L07 Ozone in the stratosphere
|
Aim: To show that the presence of ozone in the stratosphere is necessary to filter out the sun’s short wavelength UVradiation, and that unlimited use of CFC’s can result in a drastic reduction of the ozone concentration |
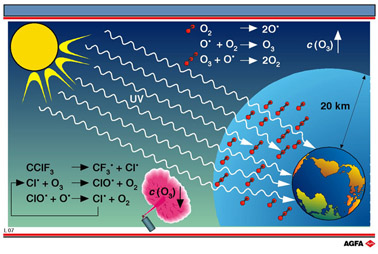
Ozone (O3) plays
a crucial role in the chemistry of the stratosphere. Almost 90% of the ozone in the
atmosphere as a whole is to be found there. There are only of the order of several tens of
molecules per million molecules of air, but nevertheless it has a major effect due to its
high reactivity and its absorption of short wavelength UV-radiation.
The concentration of ozone in the atmosphere is maintained by a balance between formation
and decomposition reactions.
1. O3 formation is a two-step. process involving the scission
of O2 into two oxygen radicals (O•), under the influence of
electromagnetic radiation (sunlight)
![]()
and addition of an oxygen radical to an oxygen molecule.z
2.
![]()
The molecule M is mostly N2 or O2. The ozone molecule is prevented
from decomposing by transferring excess energy to the molecule M.
3. Decomposition of O3 is a single-step process involving
scission upon the absorption of sunlight and reaction with oxygen radicals.
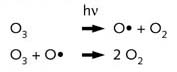
Only a small proportion of short wavelength Uvrays reach the Earth.
Other radicals also react with ozone. In the presence of such radicals, the ozone concentration in the stratosphere falls and the stratosphere can no longer fully fulfil its function as a UV-filter.
Pollution is responsible for chlorine
radicals (Cl•) and chloroxy-radicals (ClO•) in the stratosphere which destroy
ozone. These are decomposition products of ‘chlorofluorocarbons’ so-called
CFC’s. CFC’c are fairly inert materials at sea level and their property of
changing readily between gas and liquid states upon changing the pressure have led to
their use as propellants in aerosol sprays and coolants in refrigerators and freezers.
In the stratosphere, CFC’s fragment under the influence of UV-radiation from the sun
and react with (for the most part) hydroxy-radicals.
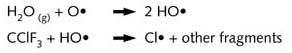
The chlorine radical can destroy a number of ozone molecules via a chain reaction:

In this way, a single chlorine radical can degrade up to 100,000 ozone molecules before
being inactivated e.g. by reacting with NO2 :
![]()
Owing to
the destruction of the ozone molecules in the stratosphere, many more
short wavelength UV-rays reach the Earth’s surface. This has a number
of harmful effects, including an increased risk of skin cancer.
For these reasons, the use of CFC’s has been severely restricted.
However, the destruction of the ozone layer will continue for some time
to come, because it will take several tens of years for all of the CFC’s
on the Earth’s surface to reach the stratosphere.
Butane gas is now frequently used as a replacement for CFC’s in aerosols.
This can be seen from the inflammability warning symbol on the can. Refrigerators
now use hydrogenated CFC’s, so-called HCFC’s, such as CHF2Cl,
as refrigerants instead of CFC’s. These HCFC’s react rapidly
with HO-radicals in the atmosphere, so that they do not reach the stratosphere.
Note :
The question is often posed as to why the thinning of the ozone layer
(the “hole”) is more marked over the South Pole. NO2
is a radical trap for Cl•, HO• and ClO• eventually forming
nitric acid (HNO3). At the extremely low temperatures of the
southern polar winter the nitric acid freezes and is no longer a source
of new
NO2-molecules.
This results in reduced trapping of these radicals and hence increased
degradation of ozone molecules.