L08 Catalytic converters in cars
Aim: To show the structure of a catalytic converter and explain which reactions happen inside it |
The large numbers of cars
in the world are causing major environmental problems. The exhaust gases contain carbon
monoxide, nitrogen oxides and unburned hydrocarbons. Leaded petrol also contains toxic
lead compounds (lead tetra-ethyl). Carbon monoxide is very poisonous, blocking the
oxygen-binding sites of haemoglobin, so that the blood can no longer transport oxygen.
Nitrogen oxides are both harmful and a source of acid rain due to nitric acid formation.
Volatile organic compounds react with nitrogen oxides under the influence of sunlight and
contribute to the formation of photochemical smog and ozone pollution, see illustration
L05.
The release of these substances has to be drastically reduced to keep the environment
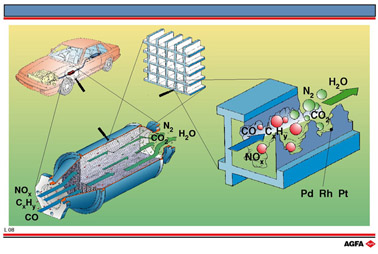
clean. One method is the use of a catalytic converter, as shown in illustration L08. A
catalytic converter consists of a stainless steel chamber containing a ceramic structure
with narrow channels lined with the three metals platinum, palladium and rhodium.
These metals act as a heterogeneous catalyst in:
- The oxidation of poisonous carbon
monoxide to carbon dioxide
- The reduction of oxides of nitrogen
to nitrogen.
- The oxidation of unburned hydrocarbons
to carbon
dioxide and water.
Lead poisons the heterogeneous catalyst and therefore only lead-free petrol can be used in cars fitted with a catalytic converter. The exhaust gasses in the catalytic converter have a temperature of 300°C and stay for only about a tenth of a second. Under these conditions the nitrogen oxides are reduced to nitrogen and the liberated oxygen oxidises the carbon monoxide and the unburned hydrocarbons. To ensure that adequate oxygen is present, the converter also contains a lambda-probe which controls the fuel/air balance so that sufficient air is present to oxidise fully the chemical species present.