L06 The greenhouse effect
Aim: To show what is understood by the “greenhouse effect” and also that it is in part necessary for life |
Many common
cultivated plants (e.g. tomatoes, orchids) require relatively high temperatures
to grow well. A greenhouse provides a simple way to capture the energy
of sunlight, providing a growth environment with an increased temperature
without the need for costly (and often environmentally unfriendly) heating
installations.
Rays of sunlight impinge upon the Earth and are absorbed by plants (red
and blue components) and by the dark-coloured soil. Both are warmed up
by the absorbed energy. However, these objects themselves also emit radiation,
as do all bodies, the wavelength of the radiation emitted being determined
by the temperature of the object. At the relatively low temperatures of
soil and plants (compared to the sun) the emitted radiation is in the
IR range (long wavelength, low energy). This re-emission will be accompanied
by cooling, so that the object will ultimately reach an equilibrium temperature.
In a greenhouse, however, a special situation arises. The glass in a greenhouse
possesses the remarkable property of being transparent to visible-light
rays (between 400 and 700 nm) while absorbing most infrared (IR) and ultraviolet
(UV-B and UV-C). Thus whilst visible light-rays of the sun can pass into
the greenhouse, the re-emitted IR radiation is reflected by the glass
into the interior and is ultimately re-absorbed.
The result is that a very high proportion of the visible light part of
the incident sunlight-energy is captured within the greenhouse. When the
sun is shining, it is always warmer inside a greenhouse than outside.
(In practice, however, much energy is lost by conduction. Although glass
is not a good conductor of heat, the area is large and its thickness small,
so that losses are substantial).
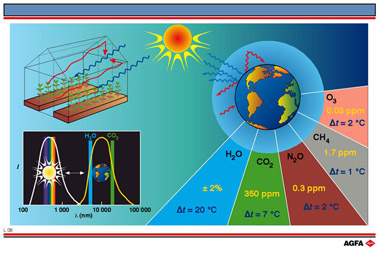
The Earth’s atmosphere, which is made up of gases, acts in a similar
way to the glass of a greenhouse. Most gases allow the passage of light-rays,
although there is always some scattering, this being greater for blue
light (short wavelength) than for red light (long wavelength). About 30%
of incident sunlight-rays are reflected by the clouds and the Earth’s
surface, another 20% is absorbed by water molecules, ozone and dust leaving
about 50% of the incoming solar energy to be absorbed by the Earth’s
surface (sea, soil, plants, etc.).
Like the plants and soil in our greenhouse, the clouds and the surfaces
of the land and sea all emit IR-waves.
Most of the IR-radiation from the surface is absorbed by the clouds, which
in turn re-emit IR-rays in all directions. Part of these rays reach the
ground and some disappears into space. This loss of energy cools the Earth’s
surface down.
Some gases are not transparent to IR-light, but absorb it. These gases
include water vapour, nitrous oxide (dinitrogen monoxide), carbon dioxide,
methane, ozone, and chlorofluorocarbons, so-called CFC’s. These gases
reemit the absorbed energy in all directions.
The high concentration of water vapour in the atmosphere results in an
increase in temperature at the Earth’s surface of about 20°C. Without
this beneficial greenhouse effect the Earth would be an ice-cold planet.
The so-called global-warming effect of these gases is shown in the table below as the degree to which a gas absorbs IR-radiation, compared to CO2. This table also shows the increase in the concentration of these gases in the atmosphere between 1800 and 1992.
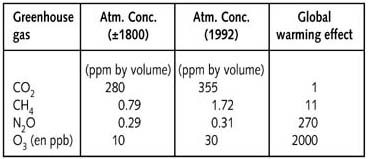
Owing to its high concentration, CO2 is the most important
greenhouse gas (after water vapour). Despite the low concentration of
CFC’s, their high global warming effect means that they contribute
about 15% to global warming. Since the concentration of carbon dioxide
has been shown to vary considerably both up and down over the history
of the earth the possible existence or not of non-natural warming of the
earth is the subject of hot debate.
One
view says that the current heating up of the earth’s surface is a
temporary phenomenon which will eventually be compensated for by the sea
and plant life; yet another says that the consequences will be much more
severe.
In the last 100 years the average temperature has risen by 0.3 to 0.6
°C, and the sea level by 10 to 20 cm.
These changes may have been caused by natural variations in the climate,
but it is also possible that natural variations are masking the deleterious
effects of the greenhouse gases.
The IPCC (Intergovernmental Panel on Climate Change) estimates that the
effect of the increase in greenhouse gases could result in an increase
in average surface temperature between 1.5 and 4.5°C.
This would result in:
- an increase
in sea level resulting in melting of the polar ice caps, expansion of
the water
- extreme
weather conditions in certain places on Earth.
The Mediterranean climate would move north; the Mediterranean area would assume an African climate. - plants growing faster, owing to the higher concentration of CO2 and the higher temperature.
Even though the consequences remain uncertain, every country is trying
to prevent the concentration of nonnatural greenhouse gases increasing
further. By the time the effect becomes clear it will already be too late
to take action. Most gases remain in the atmosphere for a long time, varying
from decades for methane to more than a hundred years for carbon dioxide
and nitrous oxide (dinitrogen monoxide).