L05 Photochemical smog
Aim: To show that the presence of NO• and volatile organic chemicals cause the O3-concentration in the atmosphere to increase |
Ozone, O3 , a reactive and irritant substance, is formed by the action of sunlight on nitrogen dioxide:
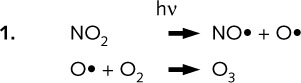
Ozone itself reacts with NO•, a less reactive radical, producing the starting
materials, NO2 and O2 again.
![]()
These reactions are thus in a state of equilibrium. This process produces a steady state
concentration of O3 that is a function of the initial concentrations of NO and
NO2.
Unless other factors interfere, the O3 concentration cannot exceed the
irritation threshold.
Under the influence of sunlight, hydroxyl radicals are formed in the atmosphere, for
example by the following reaction:
![]()
These
hydroxyl radicals are fairly active. They react with volatile organic
compounds such as methane, petrol fumes, etc. In the presence of NO•
this yields NO2, water, aldehydes and many other products without
consuming a molecule of O3.
The following is an example of such a reaction sequence:

The natural volatile organic compounds emitted from vegetation also react rapidly with the ozone and whether they are a net source of ozone or a net consumer of ozone depends on the natural volatile organic compound to NOx ratio and the sunlight intensity.
At high ratios of volatile organic
compounds to NOx, the NO2 concentration rises and
the NO concentration falls.
The increased NO2 concentration accelerates the formation of
ozone (reaction 1).
Reduced concentrations of NO• make the removal of ozone more difficult
(reaction 2).
To summarise, oxides of nitrogen, volatile organic compounds and sunlight,
are necessary to form photochemical smog.
Oxides of nitrogen are emitted by industry and traffic.
Volatile organic compounds include solvents, unburned petrol, etc. Since
sunlight is also needed, this type of airpollution is most common on windless
summer days.
One important consequence of an increased ozone concentration is its adverse
effect on the respiratory system: difficult breathing, irritation of the
bronchial passages, headaches ,etc. An increased ozone concentration also
has an adverse effect on plant life. There is not only visible damage,
but also reduced yield and slower growth.
Many materials such as elastomers, e.g. rubber tyres, textile-fibres,
paint, etc. are also oxidised faster by ozone.
The natural ozone concentration is about 60 µg/m3. The maximum
acceptable concentration over an 8-hour period has been established by
the World Health Organisation to be 110 µg/m3. This value is,
however, regularly exceeded on windless summer days.
