ST16
Separation of homogeneous solid - liquid mixtures by chromatography
| Aim:
To discuss chromatographic separation on a thin layer. |
Illustration
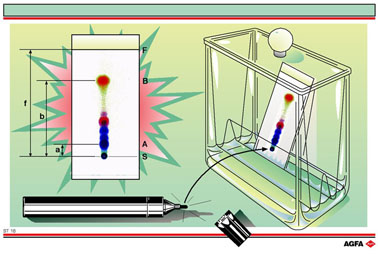
ST 16 shows one of the simplest forms of chromatography, namely thin layer
chromatography.
Chromatography is the separation of a homogeneous solid - liquid mixture,
based on differences in the equilibrium distribution of the various dissolved
substances between the two phases. In chromatography a distinction is
made between the stationary phase (the solid phase) and the mobile phase
(the elutant). Each component of the mixture undergoes an equilibrium
distribution between the two phases. A component that is to be found chiefly
in the mobile phase moves more quickly than a component that shows a greater
affinity for the stationary phase. A difference arises in the rate of
movement of the components and so these can be separated.
To start
a thin-layer chromatography experiment a small quantity of the mixture
to be analysed is put onto the thinlayer plate using a capillary tube.
The plate comprises a carrier, e.g. glass or aluminium, onto which a thin
layer of a few tenths of a millimetre is coated. This layer forms the
stationary phase and is made of either modified or non-modified silica
gel (SiO2), aluminium oxide (Al2O3) or
cellulose. Once the solvent has evaporated the sample spot is formed.
Next, the thin-layer plate is put into the developing tank.
This
tank contains a shallow layer of the mobile phase, a few millimetres deep,
so that the sample spot remains above the liquid surface. The elutant
(the mobile phase) rises as a result of the capillary action of the stationary
phase and carries the various components with it. The capillary action
is however counteracted by gravity. As a result the mobile phase moves
more and more slowly as it rises up the plate, and finally its speed falls
to zero.
The experiment is terminated long before tis situation is arrived at.
After detection, the place where a compound is found on the resulting
chromatogram is given by a socalled Rf value, defined
as :
![]()
The
different components in the mixture have different Rf
values as a result of their differing affinities for the two phases.
The Illustration shows the separation of a black ink mixture from a felt-tip
pen. The ink is first extracted from the pen using a solvent (in this
case methanol). The extract is then put onto the thin-layer plate and
eluted with an 80/20 methanol / water mixture that also contains 0.5 mol/l
sodium chloride. The various components of the black ink separate out
over a large part of the plate according to their affinities for the mobile
and the stationary phases. The results show that the black ink from a
felt-tip pen contains various dyes. The experimental Rf
value for the first dark blue spot is 0.11 ( = a/f) whilst the red spot
that is furthest away from the starting point has an Rf
value of 0.70 (b/f).
The higher the spot is transported up the plate by the mobile phase, the
greater the Rf value.
If this experiment is carried out under standard conditions it is possible
to identify the dyes from their Rf values. In the
example detection of the components is simple because we are dealing with
coloured dyes. For colourless components other identification procedures
are needed to see the spots, e.g. viewing under ultra-violet light.