ST15
Separation of gas - gas mixtures
| Aim:
To isolate nitrogen gas from air using membrane separation. |
Gas - gas
mixtures can be separated either by fractional distillation of liquified
gas mixtures, e.g. liquid air, or by passing them over a series of membrane
separators. These membranes are built of bundles of hollow polymer tubes.
Gases can diffuse through their walls. The size of the pores in the polymer
walls determines which gas particles (molecules) pass through easily and
which with more difficulty.
For
most membrane types the permeability to oxygen gas is greater than that
to nitrogen gas, seeing the differences in molecular size. The ratio of
the number of oxygen to nitrogen molecules passing through the membrane
determines the selectivity of the membrane, and should be at least four.
This ratio is called the selectivity or separation factor of the membrane.
In practice this means that the gases must pass through several membranes
in order to obtain as complete as possible a separation. The table below
gives the selectivity of various polymer types for N2/O2
gas mixtures. The speed of separation can be increased by a combination
of increased pressure (from 0.7 MPa to 10 MPa) and increased temperature
(from room temperature to 95°C). The permeability of these membranes
is always higher for oxygen gas than for nitrogen gas: an oxygen-gas molecule
has a smaller volume (22.467 Å3 ) than a nitrogen-gas
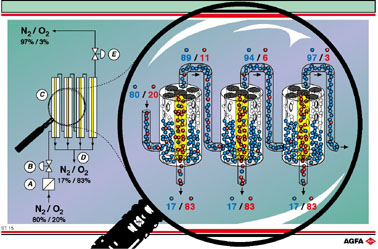
molecule (23.647 Å3 ). On the illustration, below left,
the incoming air mixture is compressed (A). The gas mixture is then let
in, through a regulator (B), to the membrane separator (C). At the bottom
of the separator (D) oxygen enriched air is discharged. At
the top of the separator almost pure nitrogen is discharged via a regulator
(E).