ST04
Separation of heterogeneous solid - solid mixtures and homogeneous solid
- liquid mixtures (solutions)
| Aim:
To illustrate extraction techniques. |
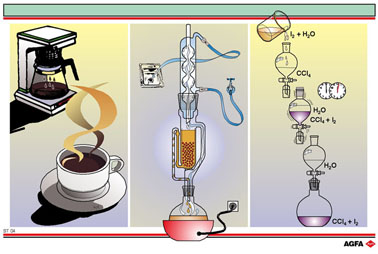
The left-hand
side of the illustration shows a coffee maker. This is a type of extraction
in which a suitable solvent (hot water, the extraction liquid) is added
to the solid mixture (ground coffee beans) in order to dissolve one or
more of the components.
The use of hot water increases the speed at which the components dissolve
and also increases the solubility of the components that are to be extracted.
In addition to the extraction step in the coffee machine, there is also
a filtration step. The coffee is thus a filtrate that can be further separated
into its solid and liquid components. If the water is evaporated off the
extracted ingredients crystallise out, this being the basis of instant
coffee.
In the middle of the illustration a Soxhlet apparatus is shown. This type of apparatus is used for continuous extraction. First the mixture, either solid - solid or solid - liquid is placed in the extraction thimble. The extraction solvent is then warmed, and rises via the right-hand side arm until it reaches the spherical condenser. Here the solvent condenses and drips back into the extraction thimble, where the substance that is to be extracted now dissolves. When the extraction-solvent has risen to the overflow-level, it flows via the siphon on the left-hand side back into the flask underneath. This process continues to run until the heater is switched off. The extracted substances are found in the flask, dissolved in the solvent.
Techniques of this type are used very frequently. They can be used to extract useful materials from plants (e.g. oils from nuts, perfume from plants etc.) and remove additives from plastics (e.g. plasticisers).
To illustrate the separation of a homogeneous solid - liquidmixture (a solution) the extraction of di-iodine from water has been chosen. Carbon tetrachloride (CCl4) is used as the extraction solvent; iodine is far more soluble in this solvent than in water. In this method a separating funnel is used so that the separation of the liquids can proceed as nearly as possible to completion. The main requirement in a separation of this sort is that the two liquids are as far as possible insoluble in one other.
The method
is as follows: a light yellow solution of diiodine in water is added to
a quantity of carbon tetrachloride in a separating funnel. The stopper
is inserted and the funnel thoroughly shaken in order to extract as much
of the iodine as possible. The funnel is placed in a ring, the stopper
removed and the mixture allowed to settle out into two layers. The top
layer is the colourless water layer and the bottom layer the purple carbon
tetrachloride layer. When the tap is opened the lower layer can be run
off as completely as possible into a recipient (here a flat-bottomed flask).
The purple colour is caused by the good solubility of the purple di-iodine
crystals in CCl4.
(Note: CCl4 is a poisonous, environmentally dangerous product.
It must never be used by students. If a teacher does the experiment, gloves
should be worn and the experiment performed in a fume cupboard!).
In general the choice of extraction solvent and extraction temperature
are very important in extraction processes. On the one hand the substance
to be extracted needs to be as soluble as possible in the solvent, to
obtain quantitative extraction. On the other hand every effort should
be made to avoid unwanted ingredients being extracted at the same time
(selectivity). When coffee is decaffeinated for example the aim is to
remove only the caffeine and as little as possible of the other ingredients
(e.g. flavour and colouring). As stated above, the temperature of the
extraction medium is very important. The solubility of solids and liquids
usually increases with temperature and this means the extraction will
proceed faster.