R16 A zinc battery and a button cell
Aim: To explain the construction and electrochemical processes of a small zinc battery and a button cell |
Illustration R16 shows two
different batteries which are in everyday use. Their operation can be compared with that
of the lead-acid accumulator (see illustration R15).
Zinc battery
This battery, which has a rather unusual construction, still carries the name of its
inventor, the French scientist Leclanché. It is the basis of the familiar 1.5 V batteries
used in torches and other household appliances requiring batteries. The cathode consists
of a central current collector (which can for example be made of copper) surrounded by a
paste of
MnO2 , KOH, ZnO and small graphite particles. The graphite
particles, which are present in high concentration, are there to conduct electrons to the
central collector. The anode, which forms the shell of the battery, is made of zinc.
During discharge of the battery this zinc layer is slowly oxidised to zinc oxide, whilst
in the cathode compartment Mn(IV) ions in MnO2 are reduced at the surface of the graphite
particles.
The two electrode-compartments are separated by waxed paper and/or wax.
The half-cell-reactions for the Leclanché cell are:

Zinc (the anode, at the top right of the table of the standard reduction potentials in
illustration R7) is the reducing agent, being oxidised to zinc ions.
The
oxidising agent is the MnO2 (in the cathode compartment) which is found at the
bottom left of a standard table of reduction potentials. Mn(IV) ions are reduced during
discharge, its oxidation number being changed from +IV to +III.
![]()
The standard reduction potentials for the two half-reactions can be used to calculate the standard reduction potential for the cell:
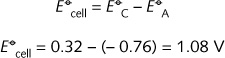
In practice the cell
potential is 1.55 V, the difference being due to non-standard concentration of ions in the
cell (see illustration R8).
This cell is non-rechargeable due to the following irreversible reactions :
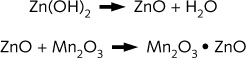
During the last few years, rechargeable batteries have been developed using a similar
construction by preventing these irreversible reactions from taking place.
Button cells.
Button cells, which are used in watches, cameras, calculators etc., work on a similar
principle. The half-cell-reactions are:
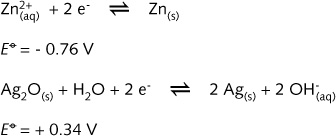
The reducing agent (anode) is once again metallic zinc.
The oxidising agent, which is contained in the cathode compartment, is silver(I) oxide
mixed with graphite particles, mercury(II) oxide, and potassium hydroxide (the
electrolyte). The overall discharge reaction is:
![]()
These batteries are small and therefore only have a small capacity.
