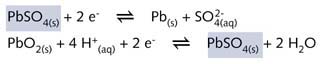R15 A lead-acid accumulator
Aim: To describe the construction, operation and characteristics of a lead-acid accumulator |
An accumulator, or in more
general terms a battery, is a classic example of the application of electrochemical
reactions. It is an electrochemical cell, which accumulates or stores electric charge. It
consists of chemical components, which function as the anode and others which function as
the cathode, connected together by an electrolyte solution or an electrolyte paste and a
porous barrier or salt bridge between the anode and cathode cells.
If a resistance, e.g. an engine, or a lamp is placed between the anode and cathode,
chemical energy will be converted into electrical energy in a self-sustaining electron
transfer reaction. An accumulator is a chemical source of electricity.
Batteries and accumulators are very much part of the modern way of life. They vary in size
from minute button batteries, such as are found in watches, to large heavy blocks, that
function as emergency electricity sources for hospitals, ships and vehicles. They are
usually made for a specific use and the lead-acid accumulator is widely used as an
electrochemical source of electricity in cars and lorries. The construction and operation
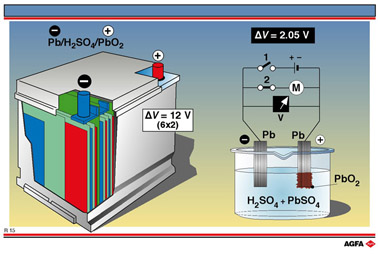
of a lead-acid battery is shown in illustration R15.
Construction of a lead-acid battery
It consists of a polypropene container, containing six lead-acid electrochemical cells,
each giving an e.m.f. of 2 V (2.05 V), connected in series. While the voltage of a
lead-acid battery is always a multiple of 2 V, its power can vary considerably. It can be
increased by dividing each cell with microporous polyethene dividers. All the anode and
cathode plates within a single cell are linked together, the anode of one cell being
linked with the anode of the next cell etc. and the cathodes of the cells being likewise
linked together.
The anodes are all made of lead and the cathodes all of solid lead(IV) oxide or lead
dioxide, which is mounted on a lead base. Both poles of the battery are also made of lead.
The electrolyte is a 80% solution of sulphuric acid saturated with PbSO4.
Electrochemistry of a lead-acid battery
Discharging :
During discharge the electrode reactions to be taken into account are, at the negative
pole (now the anode)
![]()
at the positive pole (now th ecathode)
![]()
The battery then functions as an
electrochemical cell.
Electric current is produced by the self-sustaining redox reaction between the top right
substances with the bottom left substances :
![]()
Each compartment in the battery corresponds to a cell voltage
![]()
as long as concentrations and temperature are standard.
In use, lead-acid batteries gradually become less efficient. The main redox reaction (but
also other processes) consume sulphuric acid. Since the mass density of the electrolytic
solutions increases with concentration of H2SO4, any efficiency loss will result in
a decrease in the density of the battery acid. By measuring the mass density (specific
gravity) of the electrolyte solutions, the efficiency of the battery can be checked.
Charging:
A lead-acid battery has a reasonable lifetime due to a possible reversion of the redox
reactions.
![]()
The reversed reactions
![]()
can regenerate the substances used during the discharging process. Charging is only
possible by continuing applications of a potential from an outside power source, e.g. a
car’s alternator or generator, or a charger plugged into the mains.
As long
as current is supplied to the battery, Pb2+ ions are reduced to lead metal (at
the lead electrode) while, at the PbO2 electrode, Pb2+ ions are
oxidised to PbO2. Pb and PbO2 are deposited on the electrodes.
The half-cell reactions during charging and discharging are exactly the same. The charging
process however involves the weaker oxidising agent (top left) and the weaker reducing
agent (bottom right) :