R01 - R02 non-redox reactions - redox reactions
Aim: To show that there are electrical aspects to certain chemical reactions and hence show the existence of electron transfer reactions |
Chemical
reactions can be classified into various types: synthesis, decomposition, dissociation,
replacement, precipitation, acid-base and redox to name but a few.
Some of
these types of reaction involve changes in the electron structure of the (charged) atom
others do not. e.g. (i) the dissociation in water of an ionic compound into its component
ions; and (ii) the formation of a crystalline precipitate from ions in solution. However,
they are all chemical reactions.
Non-redox reactions
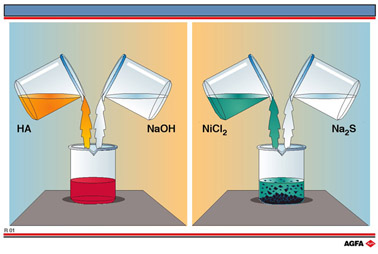
Illustration R1 depicts simple experiments illustrating two types of reaction in which
there are no changes in the electron structure of the (charged) atoms.
The
left-hand side of R1 depicts the addition of an aqueous solution of sodium hydroxide
(NaOH), which is colourless, to a solution of a weak acid, the indicator phenol red, which
is yellow coloured. Phenol red is a weak acid which dissociates in water:
![]()
If
an acid is added the reaction is displaced to the left and the yellow-coloured
HA is formed. If an alkali, e.g. sodium hydroxide, is added, the reaction
is displaced to the right and the red-coloured A- ions are
formed.
![]()
This type
of acid-base reaction is classified as a proton transfer reaction, a proton being
abstracted from HA by the OH- ions.
The right-hand side of illustration R1 depicts the reaction between an aqueous solution of
nickel chloride (NiCl2), which is green, and an aqueous solution of sodium
sulphide (Na2S), which is colourless.
When
these aqueous solutions are mixed a colour change is observed which cannot be explained by
physical dilution.
The original green colour of the nickel chloride is replaced by a less coloured liquid
with a black precipitate distributed in it. This black precipitate is nickel sulphide
(NiS).
When the two salts nickel chloride and sodium sulphide are dissolved in water, they
ionise:
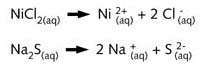
The
nickel ions (Ni2+) react with the sulphide ions (S2-)
to form crystals of nickel sulphide( NiS ), which precipitate.
This type of reaction is a precipitation reaction.
Not all reactions in which a coloured solution and a colourless solution
are mixed can be classified into proton transfer or precipitation reactions.
Redox
reactions
The
left-hand side of illustration R2 depicts a simple experiment in which
the electron structure of the (charged) atoms do change. A colourless
aqueous solution of potassium iodide(KI) is mixed with a yellow aqueous
solution of iron(III) chloride, (FeCl3). The resulting aqueous
solution is red-brown in colour.
The two salts ionise in water
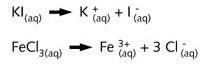
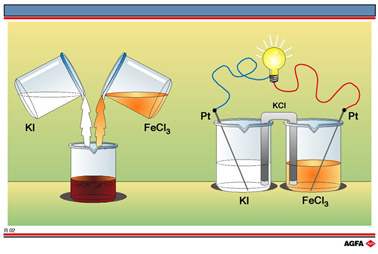
Since there is neither proton transfer nor formation of a precipitate this must belong to a different class of reaction. The type of reaction involved can be demonstrated by allowing the same reagents to react with each other in a different situation as shown on the right-hand side of illustration R2.
Here the two solutions are not mixed as in the experiment depicted on the left-hand side, but are brought into contact with each other by means of a so-called salt bridge. This allows ionic transport by cations, positively charged ions, as well as anions, negatively charged ions, without allowing the two solutions to mix. An example of a salt bridge is a U-shaped tube filled with agar gel and containing as electrolyte potassium chloride (KCl).
Inert platinum rods are placed in both solutions and these rods are in turn linked by wires to a small low voltage lamp.
An alternative experiment is to replace the lamp by an ammeter or voltmeter which enable even a small current to be detected. The current can be increased by replacing the platinum rods by platinum foil which has a larger surface area.
The glowing filament in the lamp shows that a current is flowing through the circuit i.e. that electrons are being transferred between the chemical reagents.
This type of reaction is classified as an electron transfer reaction. It can only be demonstrated in a set-up in which the reagents are not mixed. If electrodes are placed in the bottom of the beaker shown on the left-hand side of R2, no current flows between the electrodes. This does not mean that electron transfer does not occur when potassium iodide (KI) is directly reacted with iron(III) chloride (FeCl3). It only means that the electrons are not transferred to the electrodes, but directly from I- ions to iron(III) ions (Fe3+). This can be represented by:
![]()
Which itself can be represented by two half-reaction equations :
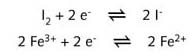
All the
electrons which are donated by the I- ions are accepted by the Fe3+
ions. In the set-up on the left, the direct electron transfer reaction, no electrons end
up in the water. They are directly exchanged from particle (ion or molecule) to particle
(molecule or ion).
In the case of a set-up such as that on the right of illustration
R2, the electrons are transferred - more slowly than in the first case
- from I- to Fe3+ via the conducting wires, through
the bulb wire.
The transport of charge throughout the salt bridge and in the solutions themselves is
carried out by different charge carriers: ions (cations as well as anions).
More details are given on illustration R3.
Moreover, the salt bridge
ensures that the two solutions do not diffuse.
This short search reveals that such special and extremely interesting chemical reactions
do exist.
However, should you think that all chemical reactions can be traced to
this one type, control tests on other reactions, such as the ones indicated
on illustration
R1, will be absolutely necessary. In set-ups analogous to the one on illustration R2 (right) no current
flow can be registered for proton transfer reactions (left on R1) or for
precipitation reactions (right on R1).