The following definitions are useful only when ![]() can be assessed unequivocally on the basis of a physica1 model of the interfacial layer, or when
can be assessed unequivocally on the basis of a physica1 model of the interfacial layer, or when ![]() can be taken as negligibly small.
can be taken as negligibly small.
Interfacial energy (![]() ) is defined by
) is defined by
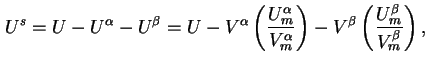
Interfacial entropy (![]() ) is defined by
) is defined by
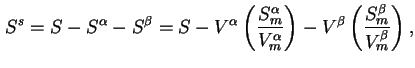
Interfacial Helmholtz energy (![]() ) is defined by
) is defined by
The corresponding quantities per unit area may be denoted by lower case letters:
Enthalpy and Gibbs energy. When the state of a system
depends upon more than one pair of conjugate mechanical (or electrical) variables, i.e. more than ![]() , then it is possible to derive several sets of functions having the character of enthalpies and Gibbs energies. These functions are related in the following way
, then it is possible to derive several sets of functions having the character of enthalpies and Gibbs energies. These functions are related in the following way
The properties of interfacial layers depend on both ![]() and
and
![]() . In defining an enthalpy in terms of the corresponding energy either
. In defining an enthalpy in terms of the corresponding energy either ![]() ,
,
![]() or
or
![]() may be subtracted from the energy function. There are thus three possible definitions of interfacial enthalpy:
may be subtracted from the energy function. There are thus three possible definitions of interfacial enthalpy:
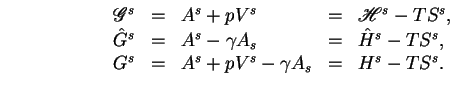
No distinguishing names have been suggested for these different functions.
A possible nomenclature, if one is needed, could be