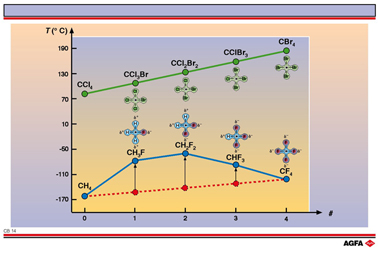
CB14 Dipole – dipole interactions influence the substances’ properties
Aim: To show that dipole – dipole interactions influence the substances’ properties |
CCl4 :
Substitution of Br for Cl in CClxBr(4-x) hardly changes the dipole
moment of the molecule. The difference in electronegativity between Cl and Br is small, so
the molecule stays more or less apolar. The boiling point increases linearly as Br is
substituted; this is to be expected since the molecular mass is also increasing.
CH4 :
Substitution of F for H has a distinct influence on the dipole moment. CH4 and
CF4 are apolar whilst CH3F, CH2F2 and CHF3
have large dipole moments. As a result the boiling points do not increase linearly as F is
substituted (red dotted line). The dipole – dipole interactions give rise to higher
boiling points than would be expected from the molecular masses.