CB13 Types of intermolecular forces
Aim: To give an overview of the most important intermolecular forces. |
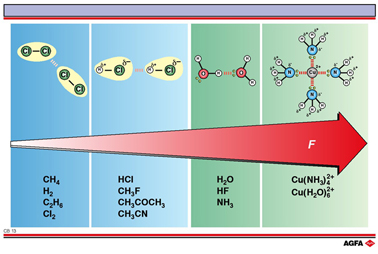
The term Vanderwaals’ forces is frequently used to describe the intermolecular forces of attraction between neutral molecules, shown in the blue area of the illustration: if present these are always the dispersion forces + dipole – dipole and other multipole interactions.
The interaction
energy of the hydrogen bond is mostly
~20 kJ mol-1, with HF2- (155 kJ mol-1)
and HCl2-, HBr2- and HI2-
(~ 50 kJ mol-1) as exceptions. This interaction energy is clearly
lower than that of the “strong” chemical bonds (100-500 kJ mol-1)
but higher than the “weak” Vanderwaals’ interactions
(0.1 – 5 kJ mol-1). Hydrogen bonds generate large, dynamic
molecular aggregates that can be broken down without losing the identity
of the basic molecule (e.g. in H2O). They play an extremely
important role in determining the physical and chemical properties of
the substance. The strength of ion-dipole interactions can be compared
with that of hydrogen bonds.