CB12 Intermolecular and intramolecular forces
Aim: To use a simple example to illustrate the difference between intermolecular and intramolecular forces. |
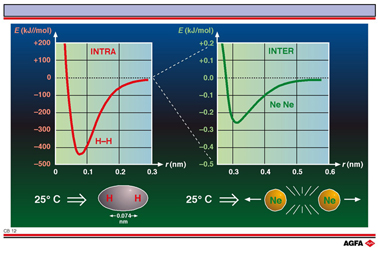
This illustration shows the interaction energy as a function of interatomic distance for H – H and Ne – Ne. Both curves show a trend consisting of attraction at large interatomic distances, and repulsion at small interatomic distances where the electron clouds of the atoms overlap each other (see illustration CB 01a).
25° C) this implies that the H-atoms are to be found in the bonded state at room temperature. H-atoms are covalently bonded and we speak of bond energy. It is an intramolecular interaction. For the Ne – Ne system the maximum interaction energy is ~ 4 x 10-22 J at 25° C (i.e. ~ 0.25 kJ mol-1), clearly lower than the available thermal energy. The interaction is too weak for the formation of stable molecules under normal circumstances but is, nevertheless, important for the physical and chemical properties. This is an intermolecular interaction.
Note that not only the energy scale but also the shape of the curve is different. For covalent chemical bonds (such as H-H) the shape of the curve can only be explained using wave mechanics whilst for ionic chemical bonds (such as Na+ Cl- ) the classic laws of electrostatics are sufficient to clarify the attraction at large distances (interaction potential ~ 1/r). For the intermolecular dispersion forces (such as in Ne – Ne) the interaction potential at large distances is proportional to 1/r6.