M11 - M12 The RUTHERFORD-BOHR atomic model
Aim: To give a simple explanation of the Rutherford-Bohr atomic model using sodium and chlorine as examples |
In the representations
![]() and
and
![]() , the
bottom number is the atomic number which can be found in the Periodic
Table. This indicates the number of protons found in the nucleus of the
atom and is also called the proton number. In a neutral atom this is equal
to the number of electrons orbiting the nucleus. Most periodic tables
also indicate the number of electrons in each orbital.
, the
bottom number is the atomic number which can be found in the Periodic
Table. This indicates the number of protons found in the nucleus of the
atom and is also called the proton number. In a neutral atom this is equal
to the number of electrons orbiting the nucleus. Most periodic tables
also indicate the number of electrons in each orbital.
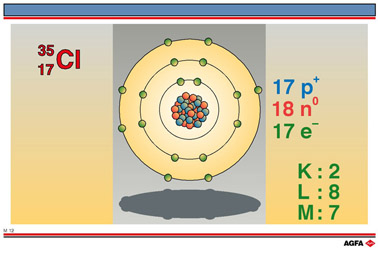
The top number is the nucleon number or mass number and gives the sum of the numbers of neutrons and protons in the nucleus.
The models shown in the illustrations are schematic, and bear no relationship to the true dimensions of the particles in the nucleus or to the diameters of the electrons orbitals (shown as concentric circles). Neither is the spatial location of the particles necessarily correct. These models are nevertheless useful when a simple method is needed to explain the formation of ionic and atomic bonds.
