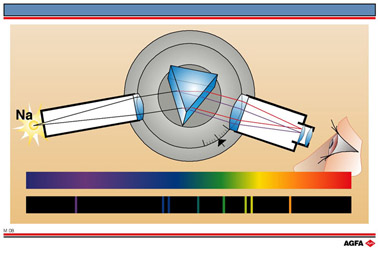
M08 Emission spectrum: a fingerprint
Aim: To show that emission spectra of gases are unique and can be used for identification purposes |
Sodium discharge lamps are, for example, frequently used for lighting purposes. These are the familiar yellow lamps seen in street lamps etc.
The uniqueness of the emission spectrum allows it to be used as the basis of a method of identification of materials. In 1868 emission spectroscopy was used to identify a previously unknown element, helium (He), in the sun’s ring. Helium was later identified in the Earth’s atmosphere.