L03 Acid rain
Aim: To show how acid rain arises, and indicate its consequences. |
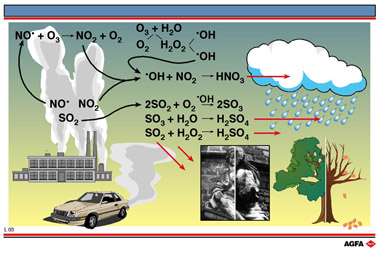
Traffic and industry emit
oxides of nitrogen (NOx) as a result of the reaction between nitrogen and
oxygen in internal combustion engines at high temperatures. Sulphur dioxide and trioxide
enter the atmosphere when sulphur containing fuels (such as coal) are burnt and when
volcanoes erupt. These gases can either be carried by the wind and deposited as acid gases
or acid particles up to hundreds of kilometres away from the point of emission or they can
be deposited as acids dissolved in rain or snow, so-called acid rain. Either form of
deposition is equally harmful to the environment.
Pure water contains equal numbers of H3O+-ions (hydrated H+
ions) and OH- ions; the pH of pure water is therefore 7. Owing to the presence
of 350 ppm of CO2 in the air, natural rainwater is always slightly acid.
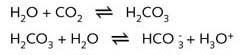
with a pH between 4.9 and 6.5. Acid rain has a higher acidity (lower pH) than natural
rainwater.
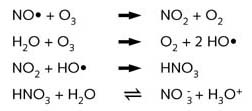
When nitric oxide, NO•, a radical, is emitted in the atmosphere it is quickly
converted to NO2. This reacts with hydroxyl radicals (HO•) giving nitric
acid.
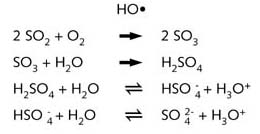
When sulphur dioxide (SO2) enters the air, it is readily oxidised in air by O2 (+ HO•) or H2O2 (see illustration “photochemical smog”)
As a result of these reactions the pH value of rain can fall to between 3 and 4 indicating increased acidity. This pH is similar to that of a solution of acetic acid. pH’s as low as 1.7 have even been measured (acid smog in Los Angeles).
Acid rain has severe environmental ramifications. Its high acidity overwhelms the buffer-capacity of the soil.
In areas with soils with insufficient buffer capacities to be able to cope with the acidity of the rainwater, the surface water very quickly becomes acidic (lakes in Northern Europe have been found to be very acidic ). Sensitive organisms, such as fish-roe, cannot survive in this environment.
If the pH of the ground falls to 3, trace elements essential to plant health, such as calcium and magnesium, and aluminium ions, which are poisonous to plants, are released from clay. Plants, and even trees, therefore die due to deprivation of essential nutrients and poisoning by aluminium ions. High concentration of aluminium ions in the lakes of Canada, Norway, Sweden, Wales and Scotland have killed the fish. The aluminium ions interfere with the operation of the fishes’ gills in such a way that they become clogged with mucus and the fish are unable to absorb sufficient oxygen to survive.
The damage to leaves and wood cannot be blamed exclusively on acid rain. Increased ozone concentrations, and an increased vulnerability to a range of sicknesses, also plays a role.
Acid rain is particularly damaging to buildings made of limestone, marble and sandstone (quartz grains held together by a coating of calcium carbonate or iron oxide) due to solubilisation of calcium carbonate as calcium hydrogen carbonate and calcium sulphate. This results in the familiar flaking away of the surface of the stone.
![]()
Silicate-based rocks are
less affected by high acidity.