E27 Is it possible to predict whether a reaction goes to completion or to an equilibrium mixture ?
Aim: To show which
thermodynamic quantities can be used to answer this question. |
Very many chemists ask the
question: “When different species react with one another, why is the reaction so
often incomplete?”
In the context of the familiar NO2-dimerization reaction, why does the reaction
end before all the NO2 is consumed?
Up to now only equilibrium reactions have been considered. What characterizes a reaction
which goes to completion ? If chlorinegas is mixed with an excess of hydrogengas, an
explosive reaction occurs in which the chlorine is completely consumed:
![]()
This
and many other reactions which go to completion are exothermic, making.
![]() negative. However, there are endothermic reactions (positive
negative. However, there are endothermic reactions (positive ![]() ) which also go to completion,
even at room temperature e.g.
) which also go to completion,
even at room temperature e.g.
![]()
There must be a more general criterion, which determines whether or not
a reaction goes to completion. Without a detailed derivation, the relationship
which determines qualitatively and quantitatively the driving force for
a particular reactant mixture is:
![]()
where ![]() H and
H and ![]() S
are the enthalpy and entropy increases associated with the reaction to
completion of the reactants respectively in a closed reaction vessel at
an absolute temperature T in kelvin.
S
are the enthalpy and entropy increases associated with the reaction to
completion of the reactants respectively in a closed reaction vessel at
an absolute temperature T in kelvin.
![]() G is known as the Gibbs
free energy change (increase).
G is known as the Gibbs
free energy change (increase).
An entropy increase in a chemical reaction occurs when the new condition
has a higher degree of disorder. This is the case for a reaction in which
molecules are formed, which are less well ordered (e.g. as a result of
phase changes) or in which the temperature has increased.
Both NO2-dimerization and NH3-synthesis result in
an increase in order!
In general the initially posed rhetorical question can be answered as
follows : “a chemical reaction in a closed reaction vessel proceeds
in that direction in which the Gibbs free energy of the reaction mixture
decreases”.
This is thus the direction in which heat is given up to the surroundings
and/or the degree of disorder increases.
According to this criterion, four types of chemical reaction can be identified
depending on the ![]() H/
H/![]() S
combination:
S
combination:
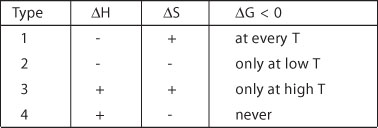
In general, reactions of
type 1 always go to completion, regardless of reaction temperature. For reactions of type
2 and 3 the position of the equilibrium is determined by the reaction temperature, the
reaction in most cases not going to completion.
NO2-dimerization and NH3-synthesis are examples of exothermic
equilibrium reactions. Endothermic equilibrium reactions of type 3 only attain a high
degree of conversion at high temperatures.
Reactions of type 4 are always associated with a positive DG,
regardless of reaction temperature. Such reactions are not necessarily impossible, but
they are never spontaneous (self-sustaining). Energy must be continuously supplied to keep
such reactions going, the electrolysis of water for example.
Reactions proceeding with a decrease in Gibbs free energy, on the other hand, proceed
without externally supplied energy.
Little has been said about the equilibrium conditions in type 2 and type 3 reactions.
The equilibrium constant K at a given temperature is related to the molar Gibbs
free energy at that temperature, by the expression:
![]()
Where R is 8.314 J/mol.K and T is the thermodynamic
(absolute) temperature in kelvin.
The quantity
![]() (the molar Gibbs free energy change at normal temperature and pressure)
can be calculated with the appropriate thermodynamic data for the reactants
and the products.
(the molar Gibbs free energy change at normal temperature and pressure)
can be calculated with the appropriate thermodynamic data for the reactants
and the products.
The enthalpy and entropy of formation at normal temperature and pressure for N2O4 and NO2 are summarized in the table below:
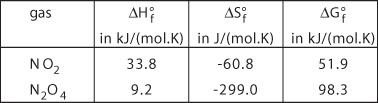
Where the subscript f stands for formation.
![]()
and K is 9.2.
The K-value obtained is a Kp-value, if the thermodynamic data concern
a gas phase reaction.
Kc can be derived from Kp by converting the
partial pressures of the gases into concentrations, as described below. The well known
relationship: pV = nRT, where p is the pressure (in Pa), V is
the volume (in m3), n is the amount of gas (in mol), R is the
molar gas constant and T is the absolute temperature (in kelvin), relates
pressure to concentration, as can be seen by rearranging the expression as follows:
![]()
If we fill in the values of the constants, then we can write down the
following expression:
![]()
Where B = 0.08206 L.bar/mol.kelvin. (For the sake of simplicity we leave out the units here.) This last equation can be used to convert Kp into Kc . The following relationship is generally valid:
![]()
Where ![]() u
is the difference in stoichiometric
numbers between the reaction products and the reactants. In the case of
the NO2-dimerization:
u
is the difference in stoichiometric
numbers between the reaction products and the reactants. In the case of
the NO2-dimerization:
![]()
For NO2-dimerization this expression gives a Kc value of 225, which agrees very well with the experimental value of 222.
How
does one predict, whether a particular reaction goes to completion or
to an equilibrium mixture?
It is evident from the above that thermodynamic quantities such as ![]() and K do not indicate whether or not a reaction goes to completion,
is fast or is slow etc...
and K do not indicate whether or not a reaction goes to completion,
is fast or is slow etc...
They do give an idea of how the degree of conversion can be optimized
or, after possible stimulation, can be made self-sustaining.
The relationship between the degree of conversion of a reaction and the
K-value depends upon the initial composition. In addition, the
expression for the equilibrium constant, K is also important.
Notwithstanding this, the table below can be used in a qualitative manner
to relate ![]() and
K to the likely type of reaction.
and
K to the likely type of reaction.
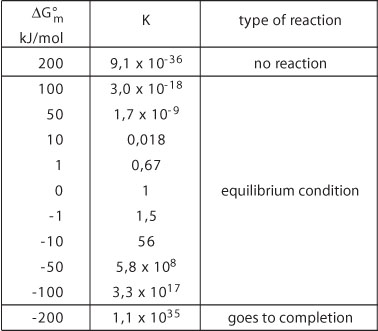
BEWARE
It is not possible to predict kinetic behaviour on the basis of such thermodynamic
data. Thus at room temperature the NO2-dimerization is not
only self-sustaining, but the reaction starts spontaneously. On the other
hand, N2 and H2 do not react at room temperature,
requiring a high temperature even in the presence of a catalyst to start
the reaction even though the ![]() -value for the ammonia
synthesis is more negative.
-value for the ammonia
synthesis is more negative.
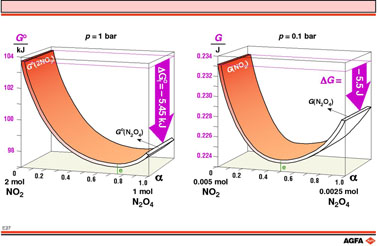
Illustration 27 shows on the left how the Gibbs free energy G°,
changes during the dimerization of NO2.
Starting with 2 moles of NO2 or 1 mole of N2O4
at room temperature and atmospheric pressure, G° decreases as
NO2 is converted into N2O4 and also as
N2O4 is dissociated into NO2 despite
G° being higher for two mol of NO2 than for a mole
of N2O4. This occurs because the entropy increases
due to the formation of a mixture, socalled “mixing entropy”.
This mixing entropy, represented by ![]() , is positive because
in a mixture there is more disorder than for a pure species. For this
mixing process
, is positive because
in a mixture there is more disorder than for a pure species. For this
mixing process ![]() is thus also negative. In this way, in this particular case, a minimum
in DG°
is attained with a mixture containing a relatively large amount of dimer.
The equilibrium is thus shifted to the right i.e. in favour of dimer formation.
In this equilibrium mixture the dimerization and dissociation reactions
have stopped and a stable minimum in G° has been reached.
is thus also negative. In this way, in this particular case, a minimum
in DG°
is attained with a mixture containing a relatively large amount of dimer.
The equilibrium is thus shifted to the right i.e. in favour of dimer formation.
In this equilibrium mixture the dimerization and dissociation reactions
have stopped and a stable minimum in G° has been reached.
However, the dependence of G upon the quantities of the components
can vary considerably upon changing the temperature or pressure from those
of the standard conditions. Such a situation is shown on the right of
illustration E27 for experiment D from illustration E06. At a pressure
of 0.1 bar, the degree of conversion (0.5) is considerably lower.