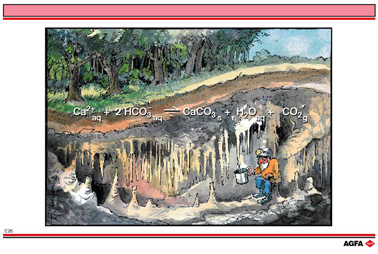
E26 Equilibrium phenomena in nature : formation of limestone caves
The equilibrium laws
derived above require that a system should be closed, so that no material exchange with
the environment can occur. For the sake of simplicity aqueous solutions in test-tubes or
beakers are regarded as closed systems. This neglects the escape of dissolved gases or the
evaporation of liquid.
In illustration E26, the result of an equilibrium mixture in a closed system evolving to
an open system is shown:
The formation of stalagtites and stalagmites in caves.
Rain-water contains some H2CO3 in addition to other acids.
Above-ground and underground the seeping water becomes more and more enriched with CO2,
particularly when passing through humus-rich soils. This increases the partial pressure of
CO2 in the water. Such water can be compared with fizzy water in a closed
bottle.
When this H2CO3 -solution comes into contact with limestone, the
following reaction takes place:
![]()
A stronger acid (H2CO3 ) can completely or partially displace a
weaker acid (HCO3- ) from its salt. In this case, it happens
together with the conversion of a weakly soluble compound into a more soluble compound.
These ions are then washed away, initially in underground rivers which flow into
above-ground rivers. This erosion process leads to the hollowing out of hollows and caves
and to hard water.
The presence of air currents and streams in the caves converts the previously closed
equilibrium system into an open system. This opening of the system has spectacular
results, due to the fairly low solubility of CO2 in water and the decrease in
CO2-solubility with decreasing temperature.
What takes place can be explained by rewriting the above equilibrium reaction as follows:
![]()
with the following expression for the equilibrium constant
![]()
which ignores the negligible solubility of CaCO3.
Stalagmites are formed in an analogous manner on the floors of caves. In both cases impressive limestone formations are produced, sometimes coloured by the presence of other precipitated salts. They are the beautiful result of a centuries’ long disturbance of a chemical equilibrium.
This phenomenon also occurs daily in our kitchens, namely the furring of saucepans, cooking equipment, on the inner walls of coffee makers, in electrical boilers, washing-up machines etc. This occurs particularly when hard water is used.
Hard water contains calcium- and magnesium-ions as well as hydrogen carbonate ions. Furring occurs particularly during the heating of hard water !
This is the reason that the use of rain-water or softened water is preferred used in washing and washing-up machines. In defurring an apparatus, a reaction is used which we have learnt from nature. A stronger acid, such as methanoic acid (formic acid) or ethanoic acid (acetic acid), is used to convert the insoluble carbonate into a soluble hydrogen-carbonate.
Similar processes take place in the kitchen, as in the use of baking powder (or sodium hydrogen carbonate). This readily dissolves in the water present in dough. Carbonic acid is formed in the slightly acidic dough:
![]()
When the dough is heated, the equilibrium shifts to the right and the equilibrium of carbonic acid with carbon dioxide and water also shifts to the right releasing carbon dioxide :
![]()
The released carbon dioxide ensures that the dough begins to rise.