E25 Industrial applications of chemical equilibria : water purification
Aim: To describe in qualitative and quantitative terms, how basic knowledge of chemical equilibria can be used in processes of direct use to us |
Potable water has become a
precious chemical material.
For many people pure water is a luxury. Others are wasteful with it. An adult requires 5 L
of potable water per day. In the West its daily consumption is much higher:
7 L for drinking and food preparation
110 L for cleaning and washing (personal, clothes, dishes, car etc.)
80 L for flushing toilets, etc.
80 L for watering grass, gardens etc.
Fortunately more and more people are coming to the view that potable water should be
exclusively used for drinking and food preparation and that rain water should be used for
other purposes. Normally water treatment for the general provision of drinking water
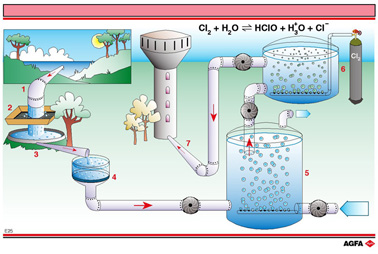
comprises a number of steps. The most important of these steps are illustrated in
illustration E25:
1. extraction from a river
2. coarse filtration to remove large objects
3. settling
4. slow filtration through sand
5. aeration
6. disinfection
7. transport to water towers
More than 90 % of the bacteria and viruses can be removed during the first five steps. The remaining bacteria can be largely made harmless by adding oxidizing agents such as chlorine (green tank on the illustration), ozone, sodium hypochlorite, calcium hypochlorite etc. In mains water and particularly in swimming pools, the addition of chlorine is evident from the smell and flavour.
3 to 6 mg of liquid Cl2 are added per litre of water.
Liquid chlorine, obtained by compressing Cl2-gas, is sold in steel bottles. Chlorine gas, a particularly apolar material, leaves the pressurized bottle as a greenish yellow gas which is absorbed by the water.
Chlorine gas is normally poorly soluble in water, but it does more than just dissolve as it reacts extremely rapidly with water. This reaction, actually an auto-oxidation reaction, is an equilibrium reaction:
![]()
The equilibrium favours the products. The HCl formed is completely dissociated.
Dissolution of chlorine makes the water more acidic.
It can therefore be assumed that the dissociation of the weak hypochlorous acid (Ka
= 3.5 x 10-8) is only slight (about 2.5 %) in a medium with a pH of about 7.
That the relatively high concentration of HClO remains undissociated is detrimentel to
bacteria, since HClO has a much stronger disinfecting power in its undissociated state. In
the treatment of swimming pool water, a HClO-concentration of 0.3 mg/L water at a pH of
7.0 to 7.7 is aimed for.
However, water treatment with chlorine gas not only guarantee disinfection to the tap, but
also hinders the growth of algae and the formation of slime in the water pipes.