

Next: ELECTROCHEMICAL TERMS IN COLLOID
Up: DEFINITIONS AND TERMINOLOGY
Previous: ATTRACTION AND REPULSION
Contents
SEDIMENTATION, CREAMING, CENTRIFUGATION AND DIFFUSION
Sedimentation volume (
 sed) or cream volume (
sed) or cream volume (
 cr) is the volume of sediment or cream formed in a suspension or emulsion. If the sediment is formed in a centrifugal field, the strength of this field should be explicitly indicated, otherwise normal gravity is understood.
cr) is the volume of sediment or cream formed in a suspension or emulsion. If the sediment is formed in a centrifugal field, the strength of this field should be explicitly indicated, otherwise normal gravity is understood.
Sedimentation equilibrium is the equilibrium between
sedimentation and diffusion.
Rate of sedimentation is the velocity of
sedimentation (
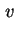 sed or
sed or 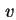 ).
).
Sedimentation coefficient (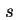 ) is the rate of
sedimentation divided by acceleration, expressed in seconds () or in Svedbergs (Sv); Sv
) is the rate of
sedimentation divided by acceleration, expressed in seconds () or in Svedbergs (Sv); Sv
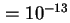 .
.
Limiting sedimentation coefficient, ![$ [s]$](img389.png) , is the
sedimentation coefficient extrapolated to zero concentration of the sedimenting component,
, is the
sedimentation coefficient extrapolated to zero concentration of the sedimenting component,
Reduced sedimentation coefficient, 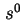 (20)25 is the sedimentation coefficient reduced to a standard temperature, usually 20 and to a standard solvent, usually water.
(20)25 is the sedimentation coefficient reduced to a standard temperature, usually 20 and to a standard solvent, usually water.
where
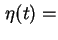 coefficient of viscosity of the solution at temperature
coefficient of viscosity of the solution at temperature 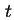 ,
,
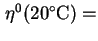 coefficient of viscosity of standard solvent at 20,
coefficient of viscosity of standard solvent at 20,
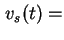 partial specific volume of sedimenting substance at temperature
partial specific volume of sedimenting substance at temperature 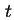 ,
,
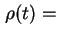 density of solution at temperature
density of solution at temperature 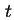 ,
,
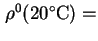 density of the standard solvent at 20.
density of the standard solvent at 20.
Reduced limiting sedimentation coefficient,
![$ [s^0(20\celsius)]$](img398.png) ,
is the reduced sedimentation coefficient extrapolated to zero concentration of the sedimenting component:
,
is the reduced sedimentation coefficient extrapolated to zero concentration of the sedimenting component:
Differential diffusion coefficient, 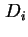 , of species
, of species 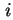 is defined by
is defined by
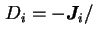
grad
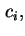
where 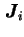 is the amount of species
is the amount of species 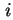 flowing through unit area in unit time and
grad
flowing through unit area in unit time and
grad 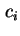 is the concentration gradient of species
is the concentration gradient of species 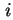 . Different diffusion coefficients may be defined depending on the choice of the frame of reference used for
. Different diffusion coefficients may be defined depending on the choice of the frame of reference used for 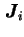 and
grad
and
grad 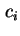 . For systems with more than two components, the flow of any component and hence its diffusion coefficient depends on the concentration distribution of all components.
. For systems with more than two components, the flow of any component and hence its diffusion coefficient depends on the concentration distribution of all components.
Limiting differential diffusion coefficient, ![$ [D_i]$](img404.png) , is the
value of
, is the
value of 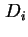 extrapolated to zero concentration of the diffusing species:
extrapolated to zero concentration of the diffusing species:
Self-diffusion coefficient, 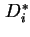 , of species
, of species 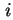 is the
diffusion coefficient in the absence of a chemical potential gradient. It is related to the diffusion coefficient
is the
diffusion coefficient in the absence of a chemical potential gradient. It is related to the diffusion coefficient 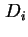 by
by
where 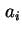 is the activity of
is the activity of 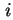 in the solution. If an isotopically labelled species (
in the solution. If an isotopically labelled species (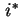 ) is used to study diffusion, the
tracer diffusion coefficient,
) is used to study diffusion, the
tracer diffusion coefficient, 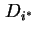 is practically
identical to the self-diffusion coefficient provided that the isotope effect is sufficiently small.
is practically
identical to the self-diffusion coefficient provided that the isotope effect is sufficiently small.
Rotational diffusion coefficient,
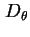 , is defined by the
equation:
, is defined by the
equation:
where
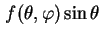 d
d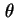 d
d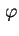 is the traction of particles whose axes make an angle between
is the traction of particles whose axes make an angle between 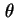 and
and
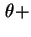 d
d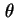 with the direction
with the direction
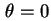 , and have an azimuth between
, and have an azimuth between 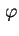 and
and
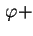 d
d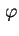 ;
;
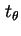 d
d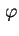 is the fraction of particles having an azimuth between
is the fraction of particles having an azimuth between 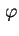 and
and
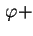 d
d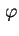 , whose axis passes from values
, whose axis passes from values 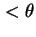 to values
to values 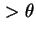 in unit time. The axis whose rotational diffusion is considered has to be clearly indicated.
in unit time. The axis whose rotational diffusion is considered has to be clearly indicated.



Next: ELECTROCHEMICAL TERMS IN COLLOID
Up: DEFINITIONS AND TERMINOLOGY
Previous: ATTRACTION AND REPULSION
Contents
2002-09-05
![]() sed) or cream volume (
sed) or cream volume (
![]() cr) is the volume of sediment or cream formed in a suspension or emulsion. If the sediment is formed in a centrifugal field, the strength of this field should be explicitly indicated, otherwise normal gravity is understood.
cr) is the volume of sediment or cream formed in a suspension or emulsion. If the sediment is formed in a centrifugal field, the strength of this field should be explicitly indicated, otherwise normal gravity is understood.
![]() sed or
sed or ![]() ).
).
![]() ) is the rate of
sedimentation divided by acceleration, expressed in seconds () or in Svedbergs (Sv); Sv
) is the rate of
sedimentation divided by acceleration, expressed in seconds () or in Svedbergs (Sv); Sv
![]() .
.
![]() , is the
sedimentation coefficient extrapolated to zero concentration of the sedimenting component,
, is the
sedimentation coefficient extrapolated to zero concentration of the sedimenting component,
![]() (20)25 is the sedimentation coefficient reduced to a standard temperature, usually 20 and to a standard solvent, usually water.
(20)25 is the sedimentation coefficient reduced to a standard temperature, usually 20 and to a standard solvent, usually water.
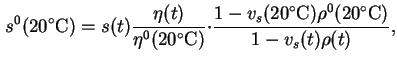
![]() ,
is the reduced sedimentation coefficient extrapolated to zero concentration of the sedimenting component:
,
is the reduced sedimentation coefficient extrapolated to zero concentration of the sedimenting component:
![]() , of species
, of species ![]() is defined by
is defined by
![]() , is the
value of
, is the
value of ![]() extrapolated to zero concentration of the diffusing species:
extrapolated to zero concentration of the diffusing species:
![]() , of species
, of species ![]() is the
diffusion coefficient in the absence of a chemical potential gradient. It is related to the diffusion coefficient
is the
diffusion coefficient in the absence of a chemical potential gradient. It is related to the diffusion coefficient ![]() by
by
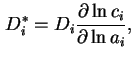
![]() , is defined by the
equation:
, is defined by the
equation: