The term colloidal refers to a state of subdivision, implying that the molecules or polymolecular particles dispersed in a medium have at least in one direction a dimension roughly between 1 nm and 1µm, or that in a system discontinuities are found at distances of that order. It is not necessary for all three dimensions to be in the colloidal range: fibers in which only two dimensions are in this range, and thin films, in which one dimension is in this range, may also be classified as colloidal. Nor is it necessary for the units of a colloidal system to be discrete: continuous network structures, the basic units of which are of colloidal dimensions also fall in this class (e.g. porous solids, gels and foams).
A colloidal dispersion is a system in which particles of colloidal size of any nature (e.g. solid, liquid or gas) are dispersed in a continuous phase of a different composition (or state).
The name dispersed phase for the particles should be used only if they have essentially the properties of a bulk phase of the same composition.
The term colloid may be used as a short synonym for colloidal system. The size limits given above are not rigid since they will depend to some extent on the properties under consideration. This nomenclature can be applied to coarser systems, especially when a gradual transition of properties is considered.
The description of colloidal systems often requires numbering of the components or constituents. It is felt that a fixed rule of numbering is unnecessarily restrictive. However, the author should make clear in all cases how he is numbering and in particular whether he is numbering by independent thermodynamic components (all neutral) or by species or constituents, of which some may be ionic, and which may be related by equilibrium conditions or by the condition of electroneutrality. In comparing English and French, it should be realized that the English word `component' is usually equivalent to the French `constituent' and the English `constituent' to the French `composant'.
A fluid colloidal system composed of two or more components may be called a sol, e.g. a protein sol, a gold sol, an emulsion, a surfactant solution above the critical micelle concentration (cf. §1.6), an aerosol.
In a suspension solid particles are dispersed in a liquid; a colloidal suspension is one in which the size of the particles lies in the colloidal range.
In an emulsion liquid droplets and/or liquid crystals are dispersed in a liquid. In emulsions the droplets often exceed the usual limits for colloids in size. An emulsion is denoted by the symbol O/W if the continuous phase: is an aqueous solution and by W/O if the continuous phase is an organic liquid (an `oil'). More complicated emulsions such as O/W/O (i.e. oil droplets contained within aqueous droplets dispersed in a continuous oil phase) are also possible. Photographic emulsions, although colloidal systems, are not emulsions in the sense of this nomenclature.
A latex (plural = latices or latexes) is an emulsion or sol in which each colloidal particle contains a number of macromolecules.
A foam is a dispersion in which a large proportion of gas by volume in the form of gas bubbles, is dispersed in a liquid, solid or gel. The diameter of the bubbles is usually larger than 1 , but the thickness of the lamellae between the bubbles is often in the usual colloidal size range.
The term froth has been used interchangeably with foam. In particular cases froth may be distinguished from foam by the fact that the former is stabilized by solid particles (as in froth-flotation q.v.) and the latter by soluble substances.
Aerosols are dispersions in gases. In aerosols the particles often exceed the usual size limits for colloids. If the dispersed particles are solid, one speaks of aerosols of solid particles, if they are liquid of aerosols of liquid particles. The use of the terms solid aerosol and liquid aerosol is discouraged. An aerosol is neither `solid' nor `liquid' but, if anything, gaseous.
A great variety of terms such as dust, haze, fog, mist, drizzle, smoke, smog are in use to describe aerosols according to their properties, origin, etc. Of these only the terms fog and smoke are included in this nomenclature.
A fog is an aerosol of liquid particles, in particular a low cloud.
A smoke is an aerosol originating from combustion, thermal decomposition or thermal evaporation. Its particles may be solid (magnesium oxide smoke) or liquid (tobacco smoke).
A gel is a colloidal system with a finite, usually rather small, yield stress. Materials such as silica gel which have passed a gel stage during preparation, are improperly called gels.
The term xerogel is used for such dried out open structures; and also for dried out compact macromolecular gels such as gelatin or rubber.
The term aerogel is used when the openness of the structure is largely maintained.
Colloidal dispersions may be lyophobic (hydrophobic, if the dispersion medium is an aqueous solution) or lyophilic (hydrophilic). Lyophilic sols are formed spontaneously when the dry coherent material (e.g. gelatin, rubber, soap) is brought in contact with the dispersion medium, hence they are thermodynamically more stable than in the initial state of dry colloid material plus dispersion medium. Lyophobic sols (e.g. gold sol) cannot be formed by spontaneous dispersion in the medium. They are thermodynamically unstable with respect to separation into macroscopic phases, but they may remain for long times in a metastable state.
Lyophilic sols comprise both association colloids in which aggregates of small molecules are formed reversibly and macromolecules in which the molecules themselves are of colloidal size.
Mixtures of lyophobic and lyophilic colloids, may form protected lyophobic colloids (cf. §1.5).
The terms lyophilic (hydrophilic, lipophilic, oleophilic, etc.) and lyophobic, (lipophobic, etc.) may also be used to describe the character of interaction of a particular atomic group with the medium. In this usage the terms have the relative qualitative meaning of `solvent preferring' (water-preferring, fat-preferring etc.) and `solvent rejecting' (water-rejecting, fat-rejecting, etc.) respectively.
The terms `solvent preferring' or `solvent rejecting' always refer to a differential process usually in the sense of preferring the solvent above itself or preferring itself above the solvent but sometimes preferring one solvent (e.g. water) above another (e.g. oil).
A colloidal electrolyte is an electrolyte which gives ions of which at least one is of colloidal size. This term therefore includes hydrophobic sols, ionic association colloids, and polyelectrolytes.
Ions of low relative molecular mass, with a charge opposite to that of the colloidal ion, are called counterions; if their charge has the same sign as that of the colloidal ion, they are called co-ions.
A polyelectrolyte is a macromolecular substance which, on dissolving in water or another ionizing solvent, dissociates to give polyions (polycations or polyanions)--multiply charged ions--together with an equivalent amount of ions of small charge and opposite sign. Polyelectrolytes dissociating into polycations and polyanions, with no ions of small charge, are also conceivable. A polyelectrolyte can be a polyacid, a polybase, a polysalt or a polyampholyte.
If all particles in a colloidal system are of (nearly) the same size the system is called monodisperse; in the opposite cases the systems are heterodisperse.
If only a few particle-sizes occur in a colloidal system the system is paucidisperse and if many particle-sizes occur polydisperse.
In heterodisperse systems the determination of particle mass or relative molecular mass gives averages, which depend on the method used. The most common averages are:
![]() ,
,
![]() , and
, and
![]() .
.
Number average relative molecular mass (= number average molecular weight)
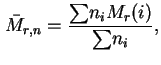
Mass average relative molecular mass (= mass average molecular weight):
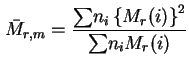
Z-average relative molecular mass (= Z-average molecular weight):
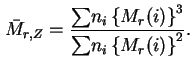
The subscript ![]() in the above definitions is generally omitted if there is no possibility of ambiguity.
in the above definitions is generally omitted if there is no possibility of ambiguity.