|
|
Vol.
30 No. 2
March-April 2008
Chemistry for Biology
by Torbjörn Norin and Upendra Pandit
The relationship between chemistry and biology is succinctly embodied in the often-cited statement “cells obey the laws of chemistry.” In this context, it is also relevant to reflect on the opening lines of the famous paper by Watson and Crick: “We wish to suggest a structure for the salt of deoxyribose nucleic acid (DNA). This structure has novel features which are of considerable biological interest” (Nature April 25, 733 [1953]). The elucidation of the structure of DNA and the understanding of its implications in the fundamental processes of life laid the foundation for the transformation of biology into a truly molecular science. An important note of caution on the interaction between chemistry and biology has been wisely expressed by Arthur Kornberg (Nobel laureate in medicine, 1959): “. . . chemistry and biology are two distinctive cultures and the rift between them is serious, generally unappreciated, and counterproductive” [Biochemistry 26, 6888 (1987)]. Fortunately, continued developments have successfully bridged the gap between chemistry and biology. Thus, the impact of genomic research has led to further erosion of the boundaries between chemistry and biology.
 |
|
IUPAC is alert to new developments in all areas in which the role of chemistry is implicated. In an earlier initiative, an interdivisional committee on biomolecular chemistry was established. Deliberations within this committee led to the IUPAC project “Chemistry for Biology.” The focus of this project was to organize a Symposium-in-Print that would illustrate the fundamental role of chemistry in a wide variety of biological topics. The project was initiated by the Division of Organic and Biomolecular Chemistry and is actively supported by a number of IUPAC divisions and standing committees. These groups have assigned representatives to the project task group in order to provide input from their specific chemical backgrounds. Some of the task group members have contributed papers to the Symposium-in-Print.
A salient project milestone was attained upon publication of a special issue of Pure and Applied Chemistry that was devoted to the subject (2007, Vol. 79, Issue 12). The contributions to this symposium deal with a broad spectrum of topics that illustrate the current scientific effervescence at the interface of chemistry and biology. On one hand, structural information at the molecular level is providing new, detailed insight into biological processes. On the other, the recognition of principles underlying biological phenomena is inspiring novel ideas for solutions to important chemical challenges.
. . . the impact of genomic research has led to further erosion of the boundaries between chemistry and biology. |
The title of the project implies a chemistry-biology relationship in which chemistry serves to provide the interpretation of biological phenomena in terms of molecular structures and chemical principles and processes. The earliest example of this relationship is provided by Friedrich Wöhler’s experiment [1828] in which he prepared the known biological substance urea by heating the abiotic compound ammonium cyanate. In his letter to Jöns Jakob Berzelius, Wöhler wrote: “I must tell you that I can prepare urea without requiring a kidney of an animal, either man or dog.” The results of this experiment triggered two important conceptual changes. It led to the demise of the theory of vital force—which was considered essential for the generation of substances of biological (i.e., natural) origin—and it represented the birth of organic chemistry as a discipline.
Scientific advances in the second half of the twentieth century have shown that as a result of the availability of structural information on biomolecules, their role in the relevant biological processes can be interpreted in terms of molecular interactions and transformations. The implication of DNA structure in the transfer of genetic information has paved the way for the elucidation of the genetic “tri-ribonucleotide” code which reveals the mechanism governing the structural link between the nucleic acids and proteins (i.e., nature’s catalysts for all biological processes). The critical molecular dimension of these relationships is emphasized by the fact that the synthesis of proteins is regulated by ribonucleic acids (RNA) and not DNA. Furthermore, chemical synthesis of the 64 possible tri-ribonucleotides established the base-sequence in the ribonucleotides that code for a specific amino acid in the synthesized protein (Holley, Khorana, Nirenberg, Nobel Prize Medicine, 1968). However, despite these brilliant illustrations of the integration of chemistry and biology, there are strong divisions between the fields as Kornberg pointed out: “chemistry and biology are two distinctive cultures and the rift between them is serious, generally unappreciated, and counterproductive” [Biochemistry 26, 6888 (1987)].
Broadly speaking, the difference between the cultures of chemistry and biology resides in their origin and approach to research. Biology has its roots in the study of natural biodiversity and of phenomena associated with biotic systems. On the other hand, the practice of chemistry is anchored in the knowledge of detailed structures, interactions, and reactions at a molecular level. It is in the latter conceptual terms that chemistry interprets biological phenomena. An essential link between the two disciplines is provided by information about the molecular structure of the relevant biological system.
Today, as structural knowledge of complex biological systems progresses, the associated biological processes enter the domain of chemical interpretation and analysis. A recent example is from the extensive and elegant structural studies of the RNA polymerase transcription machinery carried out by Roger D. Kornberg (see figure 1). Kornberg was awarded the Nobel Prize in chemistry in 2006 “for his studies of the molecular basis of eukaryotic transcription.”
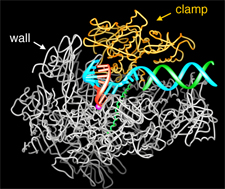 |
| Fig. 1. Structure of RNA polymerase II in the act of gene transcription. The polypeptide chain is shown in white, orange (mobile “clamp”), and green (bridge helix connecting the two largest subunits). Backbone models of the nucleic acids are shown in blue (template DNA strand), green (nontemplate DNA strand) and red (RNA). From the Nobel lecture of Professor Roger Kornberg. Reproduced by permission from the Nobel Foundation (Les Prix Nobel, The Nobel Prizes 2006, Almquist & Wiksell International, Stockholm Sweden, 2007). |
The elucidation of the sequence of three billion nucleo-base pairs of the human DNA and the sequencing of human chromosomes—as an outcome of the Human Genome project—constitutes a historical milestone. In the years to come, the analysis of genomic data, that has become available, will continue to bear fruit in many expected ways and in some yet unpredicted areas. As could be anticipated, the enhanced interaction between biology and chemistry has had an immediate impact in the area of healthcare and medicine.
Expanding knowledge about the function of protein kinases—in intracellular signal transduction and regulation of critical cellular processes—coupled with structural data, has served as a matrix for the design of clinically useful drugs. (R.P. Araujo, L. A. Liotta and E.F. Petricoin, Nature REVIEWS, Drug Discovery, 2007, Vol. 6, pp. 871-880). Also see this issue’s cover image of “The Human Kinome” (see box on page 6; courtesy of the Invitrogen Corporation). An impressive example is the development of the drug for the treatment of the haematological stem-cell disorder chronic myeloid leukemia (CML). This disorder involves translocations between chromosone 22 and chromosone 9, resulting in the abnormal BCR-ABL [breakpoint cluster region–Abelson] oncogene which codes for the tyrosine kinase responsible for CML. Treatment of CML has been sought in the development of specific tyrosine kinase inhibitors. Application of combinatorial chemistry coupled with high through-put screening has led to the development of several clinically useful drugs (e.g., imatinib, nilotinib, dasatinib) for the control of CML. A multitargeted kinase inhibitor named Sorafenib is currently being used for the treatment of kidney cancer that is resistant to interferon-alpha or interleukin-2. Sorafenib is also being studied for the potential treatment of other cancers, including melanoma, lung cancer, and mesothelioma.
In line with its mission, IUPAC advocates the creation of strong links with other disciplines so that chemistry can play a vital role in the development of multidisciplinary perspectives. In the preceding decades, the fields of medicine and agriculture have benefitted from increased interdisciplinary interaction and coordination between chemistry and biology. While IUPAC maintains its focus on fundamental chemistry and its applications, it is alert to the emergence of new interdisciplinary areas where its relevant divisions can provide a constructive platform for rapid information-exchange and cooperative undertakings. In the context of developments at the interface of biology and chemistry, closer interaction with other international scientific organizations, such as the International Union for Biochemistry and Molecular Biology, the International Union of Pure and Applied Biophysics, and the International Society for Chemical Ecology, is a logical evolution within the framework of IUPAC.
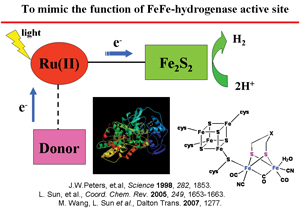 |
| Fig. 2. Catalysts, which mimic the function of FeFe-hydrogenase active site, may have many potential applications (fuel cell technology,
hydrogenation catalysts, hydrogen production). Slide from a lecture, provided by Lichen Sun (Royal Institute of Technology, Chemistry Department, Stockholm, Sweden). |
While noting the implications of chemistry for biology, it is equally important to recognize the impact of biology on chemistry. An understanding of biological interactions and phenomena leads to insights into nature’s evolutionary patterns and designs, which, in turn, can be translated into useful applications. Thus, biological research in areas of environmental science, ecology, and in agriculture and forestry, besides being fundamentally inspirational, provides exciting stimulation for investigations directed at chemically oriented goals. Current research on photosynthesis is an excellent illustration of the beneficial impact of the two-way flow of scientific information. The photosynthetic machinery of green plants has been used as a guiding concept in the development of photosynthetic molecular-devices that are capable of splitting water and generating hydrogen; for a recent example, see figure 2. Today, applications of cross-fertilization between biology and chemistry are finding expression in an increasingly expanding domain.
Disciplines in science evolve in time and new terms emerge that more adequately cover the the disciplinary landscape. Today, the multidisciplinary area in which biological phenomena and biological processes are being defined in terms of detailed structural and mechanistic events is recognized as a rapidly emerging field at the interface of chemistry and biology. IUPAC responds in an effective manner to new developments in which the role of chemistry is implicated. In an early initiative, the scope of two of the IUPAC divisions of basic chemistry—Organic and Physical Chemistry—was expanded to include activities directed at understanding the chemical basis of biological phenomena.
The term coined by IUPAC for the interface between chemistry and biology is biomolecular chemistry; that is, the chemistry of biomolecules—from the humble urea to highly complex biological systems that can be defined in molecular language. It should be added that several other terms have come into use to define the field (e.g., biological chemistry, chemical biology, chemistry-biology interface, and chemistry and biology). The undersigned authors wish to emphasize that because the term biomolecular chemistry restricts itself to the chemistry of biomolecules, it more accurately defines the interface area. Furthermore, the term biomolecular chemistry acknowledges the unique role of biology in the study and the understanding of such biological phenomena, which, due to its level of complexity, cannot to date be described in detailed molecular terms.
Torbjörn Norin <[email protected]> is an emeritus professor at the Royal Institute of Technology (KTH), Stockholm, Sweden, and a former president of the IUPAC Division of Organic and Biomolecular Chemistry (2000–2001). He is the chairman of the task group for the current interdivisional IUPAC project on Chemistry for Biology. Upendra Pandit <[email protected]> is an emeritus professor at the University of Amsterdam, The Netherlands, and a former president of the IUPAC Division of Organic and Biomolecular Chemistry (1998–1999). He is a task group member of the current interdivisional IUPAC project on Chemistry for Biology.
“Chemistry for Biology”—a symposium-in-print
Pure and Applied Chemistry 2007, Vol. 79, Issue 12, pp. 2179-2366
www.iupac.org/publications/pac/79/12/
Page last modified 19 March 2008.
Copyright © 2003-2008 International Union of Pure and Applied Chemistry.
Questions regarding the website, please contact [email protected] |