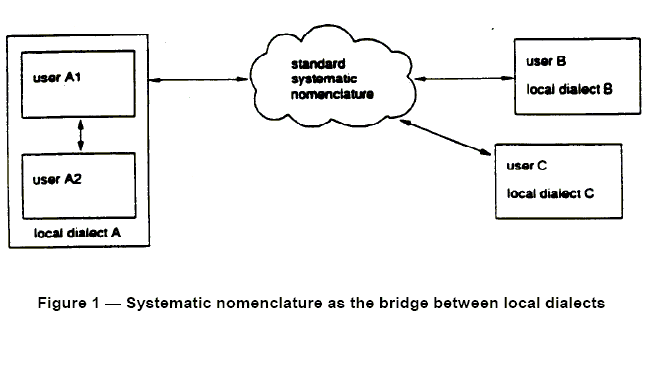
Fig. 1. From ENV 1614:1995
IV. PROPERTIES AND THEIR CODE VALUES
(IUPAC Technical report 1997 - IFCC Recommendation 1997)
Prepared for publication by:
H. OLESEN1, D. KENNY2, I. BRUUNSHUUS1, I. IBSEN1 , K. JěRGENSEN3, R. DYBKĂR4, X. FUENTES-ARDERIU5, G. HILL6, P. SOARES DE ARAUJO7, C. McDONALD8
Chairman: 1989-1995 H. Olesen (Denmark); 1996 - D Kenny (Ireland). Members: X. Fuentes-Arderiu (Spain; 1991-1997); J.G. Hill (Canada; 1987-1997); D. Kenny (Ireland; 1994-1997); H. Olesen (Denmark; 1985-1995); PL Storring (United Kingdom; 1989-1995); P Soares de Araujo (Brazil; 1994-1997); RenÚ DybkŠr (Denmark; 1996-1997); Clem McDonald (USA; 1996-1997).
Please forward comments to:
H. Olesen, Dept. Clin. Pharmacol. Q 75.2.1, Copenhagen
University Hospital (Rigshospitalet), 20 Tagensvej, DK-2200 Copenhagen,
Denmark.
Fax: +45 35 45 27 45; e-mail: [email protected]
or
[email protected]
Synopsis
To facilitate and to assure correct electronic transmission of request
and report on clinical laboratory properties over cultural and linguistic
barriers, a systematic nomenclature has been prepared for a series of laboratory
specialities.
Each defined property has been given a unique code value preceded by
the coding scheme identifier: NPU.
The NPU code value and its adhering code value string for each term
allow expression of the concept according to local script, idiom or conventions.
The coding scheme is accessible on Internet from C-NPU Home page address
http://www.ifcc-iupac.suite.dk
Scope
The coding scheme prepared is intended as a repository of code values
and terms of properties to be used in the transfer of information on such
properties through computing and telecommunication equipment used in the
health services.
Preface
The document is the fourth part of a series on properties measured
in the clinical laboratory sciences initiated in 1987.
The series will comprise the five general parts (I-IV and XI) and a
series of special parts:
| I | Syntax and semantic rules |
| II | Kinds-of-property |
| III | Elements (of properties) and their code values |
| IV | Properties and their code values (this document) |
| V | Properties and units in Thrombosis and Haemostasis |
| VI | Properties and units in IOC prohibited drugs |
| VII | Properties and units in Inborn Errors of Metabolism |
| VIII | Properties and units in Clinical Microbiology |
| IX | Properties and units in Trace Elements |
| X | Properties and units in General Clinical Chemistry |
| XI | Coding systems - structure and guidelines |
| XII | Properties and units in Clinical Pharmacology and Toxicology |
| XIII | Properties and units in Reproduction and Fertility |
| XIV | Properties and units in Tumor Markers |
| XVI | Properties and units in Clinical Allergology |
The size and complexity of part III and IV is such that their lists
will be presented in electronic format only.
The overall aim is access by electronic media of:
"Compendium of terminology and nomenclature of properties in clinical
laboratory sciences".
"Glossary of terms in quantities and units in clinical chemistry".
"Properties and units in the clinical laboratory sciences" (the present
series of documents).
Introduction
The variety of properties observed in the domain of the Clinical Laboratory
Sciences is well over 5000. The number of properties observed is 5 to 10
per inhabitant per year in industrialised countries.
An increasing part of the billions of requests and reports is transmitted
by electronic means, mostly by code values from a local coding scheme.
The expression of the meaning of a code value is according to local
habit, rules and conventions. This often is not readily transformed to
coded sets from other coding schemes.
To facilitate inter region electronic communication, the European standard
ENV 1614:1995 has presented a system of concepts based on for a systematic
nomenclature to function as a bridge between local dialects.

Fig. 1. From ENV 1614:1995
Based on this system of concepts and on the European standard ENV 12435:1996,
that deals with the presentation of results, terms have been elaborated
and codified for communication between clinical laboratory information
systems and other health information systems.
A basic document for further description and clarification is "Compendium
of terminology and nomenclature of properties in the clinical laboratory
sciences", while details of the syntax and semantic rules are given in
Ref.1.
Systematic names and results
The outcome of a laboratory examination may be schematised as
Property = Result
or more specified
System(specification)--; kind-of-property(specification) = value Ě unit
or as generally in Clinical Laboratory Sciences
System(specification)-Component(specification); kind-of-property(specification) = value Ě unit
Although the system of concepts is generally applicable, modifications
and extensions are needed in some subject fields.
For this reason, detailed coding schemes for some categories of properties
have been worked out by authorities in that speciality for publication
in separate documents. The present document is a compilation of terms from
the different specialities.
Each term has been given a code value prefixed by the coding scheme
identifier NPU (from Nomenclature, Properties and Units).
The numeric value is a consecutive number with no meaning per se. It represents
a code value string for that property; that is a series of values representing
semantic links and a series representing values for elements from the coding
scheme in part III (Elements (of properties) and their code values) (Table
1).
| Table 1. | |||
| Semantic link | Element (example) | ||
| Code value | Meaning
Now follows a code value of a: |
Code value | Meaning |
| QU59901 | System | MSHD010949 | Plasma |
| QU59902 | Specification to a system | ||
| QU59903 | Component | CAS50-99-7 | Glucose |
| QU59904 | Specification to a component | ||
| QU59905 | Kind-of-property | QU50003 | substance concentration |
| QU59906 | Specification to a property | ||
| QU59907 | Numerical value | = | 5,6 |
| QU59909 | Unit | QU09408 | millimole/litre |
| QU59910 | End code | QU99999 | End |
or as a code value string:
[NPU02192] QU59901:MSHD010949:QU59903:CAS50-99-7:QU59905:QU50003:QU59907: = 5,6:QU59909:QU09408:QU59910:QU99999:
The coding scheme for properties basically comprises a list of coded sets that is an NPUXXXXX and a corresponding code value string (Sample page 1). On expressing the code values for elements in a particular language, the list of properties is generated in that language. The present list is presented in English (Sample pages 2 and 3).
Details are in part XI Coding systems - Structure and guidelines.
Each entry comprises a term with an NPU code value and when appropriate a molar mass, calibrator, authority, (when appropriate other terms and notes), and an example in a short form.
EXAMPLE
Plasma-
Glucose;
substance concentration
millimole/litre
M = 180,2 g/mol
Authority: CAS50-99-7
[NPU02192]
P-Glucose; subst.c. = ? mmol/l
Functionality
Specialities
Testing and inclusion in the list has been done for clinical allergology,
clinical bacteriology, clinical chemistry, clinical pharmacology, complement
factors, haematology, clinical immunology, inborn errors of metabolism,
reproduction and fertility, trace elements, and thrombosis and haemostasis.
The list is to be expanded as needs arise.
Linguistic expression
The basis of the coding scheme is the code value strings, representing
the meaning of the NPU code values.
The expression has been successfully tested in:
P-Glukos; subst konc = 5,6 mmol/L Swedish
Pla-Glucosa; c.sust. = 5,6 mmol/l Spanish
Internet access
The information on properties is given in three forms on the Home page:
[1] IUPAC-IFCC (International Union of Pure and Applied Chemistry- International Federation of Clinical Chemistry), Commission/Committee on Quantities and Units (in Clinical Chemistry). Properties and units in the clinical laboratory sciences. I. Syntax and semantic rules. Recommendations 1995. Prepared for publication by Olesen H. Pure & Appl Chem 1995; 67: 1563-74; Eur J Clin Chem Clin Biochem 1995; 33: 627-36; Clin Chim Acta 1996; 245: S5-S21.
[2] IUPAC-IFCC (International Union of Pure and Applied Chemistry- International Federation of Clinical Chemistry), Commission/Committee on Nomenclature, Properties and Units. Properties and units in the clinical laboratory sciences. II. Kinds-of-property. Recommendations 1996. Prepared for publication by Olesen H, Kenny D. Eur J Clin Chem Clin Biochem 1997; 35: 317-44.
[3] ISTH (International Society on Thrombosis and Haemostasis), Scientific and Standardization Committee and IUPAC-IFCC (International Union of Pure and Applied Chemistry-International Federation of Clinical Chemistry), Commission/Committee on Quantities and Units(in Clinical Chemistry). Recommendations 1995. Properties and units in the clinical laboratory sciences. V. Properties and units in Thrombosis and Haemostasis. Eur J Clin Chem Clin Biochem 1995; 33: 637-60; Clin Chim Acta 1996; 245: S23-S28; Pure and Appl Chem 1997; 69: 1043-79.
[4] IUPAC-IFCC (International Union of Pure and Applied Chemistry- International Federation of Clinical Chemistry), Commission/Committee on Nomenclature, Properties and Units. Properties and units in the clinical laboratory sciences. VI. Properties and units in IOC prohibited drugs. Recommendations 1997. Prepared for publication by Olesen H, Cowan D, Bruunshuus I, Klempel K, Hill G. Eur J Clin Chem Clin Biochem 1997, in press. Pure and Appl Chem 1997; 69: 1081-1136.
[5] IUPAC-IFCC (International Union of Pure and Applied Chemistry- International Federation of Clinical Chemistry), Commission/Committee on Quantities and Units (in Clinical Chemistry), 1995. Compendium of terminology and nomenclature of properties in clinical laboratory sciences. JC Rigg, SS Brown, R Dybkaer, H Olesen. Oxford: Blackwell Science, 290 pages.
[6] IUPAC-IFCC (International Union of Pure and Applied Chemistry- International Federation of Clinical Chemistry), Commission/Committee on Quantities and Units(in Clinical Chemistry), 1995. Glossary of terms in quantities and units in clinical chemistry. Prepared for publication by Lehmann HP, Fuentes-Arderiu X, Bertello, LF. Pure and Appl Chem 1996; 68: 957-1000. Biochim Clin 1995; 19: 471-502.
[7] CEN/TC 251, 1995. European Standard ENV 1614. Medical informatics. Structure for nomenclature, classification and coding of properties in clinical laboratory sciences.
[8] IUPAC-IFCC (International Union of Pure and Applied Chemistry-International Federation of Clinical Chemistry), 1967. Quantities and Units in Clinical Chemistry. Including Recommendation 1966. Prepared for publication by DybkŠr R, J°rgensen K. Copenhagen: Munksgaard, 102 pp.
[9] CEN/TC 251, 1996. European Standard ENV 12435. Medical informatics. Expression of the results of measurement in health sciences.
SAMPLE PAGE 1 (code value strings)
| NPU1001 | QU59901:MSH94D014556:QU59903:CAS37517-30-9:QU59905:QU50081:QU59906:QU50601:QU59907: = ?:QU59910:QU99999 |
| NPU1002 | QU59901:MSH94D014556:QU59903:CAS37517-30-9:QU59905:QU50081:QU59906:QU50603:QU59907: = ?:QU59910:QU99999 |
| NPU1003 | QU59901:MSH94D014556:QU59903:CAS37517-30-9:QU59905:QU50003:QU59907: = ?:QU59909:QU10408:QU59910:QU99999 |
| NPU1004 | QU59901:MSH94D010949:QU59903:CAS61-00-7:QU59905:QU50003:QU59907: = ?:QU59909:QU10408:QU59910:QU99999 |
| NPU1005 | QU59901:MSH94D001769:QU59903:CAS75-07-0:QU59905:QU50003:QU59907: = ?:QU59909:QU10408:QU59910:QU99999 |
| NPU1006 | QU59901:MSH94D014556:QU59903:CAS75-07-0:QU59905:QU50003:QU59907: = ?:QU59909:QU10408:QU59910:QU99999 |
| NPU1007 | QU59901:MSH94D014556:QU59903:CAS59-66-5:QU59905:QU50081:QU59906:QU50601:QU59907: = ?:QU59910:QU99999 |
| NPU1008 | QU59901:MSH94D014556:QU59903:CAS59-66-5:QU59905:QU50081:QU59906:QU50603:QU59907: = ?:QU59910:QU99999 |
| NPU1009 | QU59901:MSH94D014556:QU59903:CAS59-66-5:QU59905:QU50003:QU59907: = ?:QU59909:QU10408:QU59910:QU99999 |
| NPU1010 | QU59901:MSH94D002555:QU59903:MSH94D000090:QU59905:QU50003:QU59907: = ?:QU59909:QU10408:QU59910:QU99999 |
| NPU1011 | QU59901:MSH94D010949:QU59903:MSH94D000090:QU59905:QU50003:QU59907: = ?:QU59909:QU09408:QU59910:QU99999 |
| NPU1012 | QU59901:MSH94D014556:QU59903:MSH94D000090:QU59905:QU50003:QU59906:QU70500:QU59907: = ?:QU59909:QU09408:QU59910:QU99999 |
| NPU1013 | QU59901:MSH94D010949:QU59903:QU60505:QU59905:QU50032:QU59906:QU70724:QU59906:QU70500:QU59907: = ?:QU59909:QU10417:QU59910:QU99999 |
| NPU1014 | QU59901:MSH94D000653:QU59903:EC2.3.1.9:QU59905:QU50032:QU59906:QU70724:QU59906:QU70500:QU59907: = ?:QU59909:QU10417:QU59910:QU99999 |
| NPU1015 | QU59901:QU65001:QU59903:EC2.3.1.9:QU59905:QU50033:QU59906:QU70724:QU59906:QU70500:QU59907: = ?:QU59909:QU10416:QU59910:QU99999 |
| NPU1016 | QU59901:QU65003:QU59903:EC2.3.1.9:QU59905:QU50033:QU59906:QU70724:QU59906:QU70500:QU59907: = ?:QU59909:QU10416:QU59910:QU99999 |
| NPU1017 | QU59901:QU65004:QU59903:EC2.3.1.9:QU59905:QU50033:QU59906:QU70724:QU59906:QU70500:QU59907: = ?:QU59909:QU10416:QU59910:QU99999 |
| NPU1018 | QU59901:QU65011:QU59903:EC2.3.1.9:QU59905:QU50033:QU59906:QU70724:QU59906:QU70500:QU59907: = ?:QU59909:QU10416:QU59910:QU99999 |
| NPU1019 | QU59901:MSH94D010949:QU59903:EC2.3.1.9:QU59905:QU50032:QU59906:QU70724:QU59906:QU70500:QU59907: = ?:QU59909:QU10417:QU59910:QU99999 |
| NPU1020 | QU59901:QU65021:QU59903:EC2.3.1.9:QU59905:QU50033:QU59906:QU70724:QU59906:QU70500:QU59907: = ?:QU59909:QU10416:QU59910:QU99999 |
| NPU1021 | QU59901:QU65023:QU59903:EC2.3.1.9:QU59905:QU50033:QU59906:QU70724:QU59906:QU70500:QU59907: = ?:QU59909:QU10416:QU59910:QU99999 |
| NPU1022 | QU59901:MSH94D014556:QU59903:QU60438:QU59905:QU50003:QU59907: = ?:QU59909:QU10408:QU59910:QU99999 |
SAMPLE PAGE 2 (full text, according to sample page 1; NPU01001 to NPU01016)
Urine-
Acebutolol;
arbitrary concentration(IOC Screen;
0 1)
M = 336,43 g/mol
Authority: IOC; IFCC/C-LDA; INN; CAS37517-30-9
[NPU01001]
U-Acebutolol; arb.c.(IOC Screen; 0
1) = ?
Urine-
Acebutolol;
arbitrary concentration(IOC Confirm;
0 1)
M = 336,43 g/mol
Authority: IOC; IFCC/C-LDA; INN; CAS37517-30-9
[NPU01002]
U-Acebutolol; arb.c.(IOC Confirm;
0 1) = ?
Urine-
Acebutolol;
substance concentration
micromole/litre
M = 336,43 g/mol
Authority: INN; CAS37517-30-9
[NPU01003]
U-Acebutolol; subst.c. = ? Ámol/l
Plasma-
Acepromazine;
substance concentration
micromole/litre
M = 326,47 g/mol
Authority: INN; CAS61-00-7
[NPU01004]
P-Acepromazine; subst.c. = ? Ámol/l
Blood-
Acetaldehyde;
substance concentration
micromole/litre
M = 44,05 g/mol
Authority: CAS75-07-0
[NPU01005]
B-Acetaldehyde; subst.c. = ? Ámol/l
Urine-
Acetaldehyde;
substance concentration
micromole/litre
M = 44,05 g/mol
Authority: CAS75-07-0
[NPU01006]
U-Acetaldehyde; subst.c. = ? Ámol/l
Urine-
Acetazolamide;
arbitrary concentration(IOC Screen;
0 1)
M = 222,25 g/mol
Authority: IOC; IFCC/C-LDA; INN; CAS59-66-5
[NPU01007]
U-Acetazolamide; arb.c.(IOC Screen;
0 1) = ?
Urine-
Acetazolamide;
arbitrary concentration(IOC Confirm;
0 1)
M = 222,25 g/mol
Authority: IOC; IFCC/C-LDA; INN; CAS59-66-5
[NPU01008]
U-Acetazolamide; arb.c.(IOC Confirm;
0 1) = ?
Urine-
Acetazolamide;
substance concentration
micromole/litre
M = 222,25 g/mol
Authority: INN; CAS59-66-5
[NPU01009]
U-Acetazolamide; subst.c. = ? Ámol/l
Cerebrospinal fluid-
Acetoacetate;
substance concentration
micromole/litre
Authority: MSHD000090
[NPU01010]
Csf-Acetoacetate; subst.c. = ? Ámol/l
Plasma-
Acetoacetate;
substance concentration
millimole/litre
Authority: MSHD000090
[NPU01011]
P-Acetoacetate; subst.c. = ? mmol/l
Urine-
Acetoacetate;
substance concentration(procedure)
millimole/litre
Authority: MSHD000090
[NPU01012]
U-Acetoacetate; subst.c.(proc.) =
? mmol/l
Plasma-
Alkaline phosphatase, liver type;
catalytic-activity concentration(37
░C; procedure)
microkatal/litre
[NPU01013]
P-Alkaline phosphatase, liver type;
cat.c.(37 ░C; proc.) = ? Ákat/l
Amniotic fluid-
Acetyl-CoA acetyltransferase;
catalytic-activity concentration(37
░C; procedure)
microkatal/litre
Authority: EC2.3.1.9
[NPU01014]
Amf-Acetyl-CoA acetyltransferase;
cat.c.(37 ░C; proc.) = ? Ákat/l
Amniotic fluid cells(cultured) protein-
Acetyl-CoA acetyltransferase;
catalytic-activity content(37 ░C;
procedure)
microkatal/kilogram
Authority: EC2.3.1.9
[NPU01015]
Amf cells(cult.) prot.-Acetyl-CoA
acetyltransferase; cat.cont.(37 ░C; proc.) = ? Ákat/kg
Chorionic villus cell protein-
Acetyl-CoA acetyltransferase;
catalytic-activity content(37 ░C;
procedure)
microkatal/kilogram
Authority: EC2.3.1.9
[NPU01016]
Chor.villus cell prot.-Acetyl-CoA
acetyltransferase; cat.cont.(37 ░C; proc.) = ? Ákat/kg
SAMPLE PAGE 3 (selected random full text examples)
Amniotic fluid-
Acetylcholinesterase;
catalytic-activity concentration(37
░C; procedure)
microkatal/litre
Other term(s): AChE; Cholinesterase;
Choline esterase I; True cholinesterase
Authority: EC3.1.1.7
[NPU01034]
Amf-Acetylcholinesterase; cat.c.(37
░C; proc.) = ? Ákat/l
Chorionic villus cell protein-
N-
Acetylgalactosamine-4-sulfatase;
catalytic-activity content(37 ░C;
procedure)
microkatal/kilogram
Other term(s): Arylsulfatase B; Chondroitinase;
Chondroitinsulfatase; Chondrosulfatase
Authority: EC3.1.6.12
[NPU01039]
Chor.villus cell prot.-N-Acetylgalactosamine-4-sulfatase;
cat.cont.(37 ░C; proc.) = ? Ákat/kg
Patient(Urine)-
Adrenalinium+noradrenalinium excretion;
substance rate(procedure)
micromole/day
Other term(s): Catecholamines; Levarterenol
[NPU01105]
Pt(U)-Adrenalinium+noradrenalinium
excretion; subst.rate(proc.) = ? Ámol/d
Urine-
Adrenergic beta-Antagonist;
arbitrary concentration(list; procedure)
Other term(s): Beta-Antagonist, adrenergic;
Beta-Adrenergic receptor blockader; Beta-Adrenergic blocking agent; Beta-blocker,
adrenergic;
Beta-blocking drug
Authority: MSHD000319
[NPU04413]
U-Adrenergic beta-Antagonist; arb.c.(list;
proc.)
[NPU04576] U-Acebutolol; arb.c.(proc.)
= ?
[NPU04577] U-Alprenolol; arb.c.(proc.)
= ?
[NPU04579] U-Atenolol; arb.c.(proc.)
= ?
Hair-
Arsenic;
substance content
micromole/kilogram
M = 74,92
Authority: IUPAC/VII/C-TOX; CAS7440-38-2
[NPU01307]
Hair-Arsenic; subst.cont. = ? Ámol/kg
Plasma-
Borrelia burgdorferi antibody(Immunoglobulin
M);
arbitrary substance concentration(procedure)
arbitrary unit/litre
[NPU08018]
P-Borrelia burgdorferi antibody(IgM);
arb.subst.c.(proc.) = ? arb.unit/l
Plasma(venous Blood)-
Carbon dioxide(total);
substance concentration
millimole/litre
M = 44,01 g/mol
Authority: IFCC/C-BGE; CAS124-38-9
[NPU01472]
P(vB)-Carbon dioxide(tot.); subst.c.
= ? mmol/l
Plasma-
Coagulation factor XIII;
relative substance concentration(immunological;
actual/norm; procedure)
M = 320 000 g/mol
Other term(s): Fibrin stabilizing
factor; Fibrinoligase; Fibrinase Laki-Lorand factor; Plasma transglutaminase;
Plasma transamidase;
Protransglutaminase
Authority: ISTH/SSC93; CAS9013-56-3
[NPU01673]
P-Coagulation factor XIII; rel.subst.c.(imm.;
actual/norm; proc.) = ?
System-
Streptokinase;
arbitrary substance concentration(IS
62/7; procedure)
international unit/litre
M = 47 408 g/mol
Calibrator: WHO 1st IS 62/7
Other term(s): SK; STK
Authority: CAS9002-01-1
[NPU04024]
Syst.-Streptokinase; arb.subst.c.(IS
62/7; proc.) = ? int. unit/l
SAMPLE PAGE 4 (short form - abbreviated presentation - according
to sample pages 1 and 2)
| NPU | Property |
|
01001
|
U-Acebutolol; arb.c.(IOC Screen; 0 1) = ? |
|
01002
|
U-Acebutolol; arb.c.(IOC Confirm; 0 1) = ? |
|
01003
|
U-Acebutolol; subst.c. = ? Ámol/l |
|
01004
|
P-Acepromazine; subst.c. = ? Ámol/l |
|
01005
|
B-Acetaldehyde; subst.c. = ? Ámol/l |
|
01006
|
U-Acetaldehyde; subst.c. = ? Ámol/l |
|
01007
|
U-Acetazolamide; arb.c.(IOC Screen; 0 1) = ? |
|
01008
|
U-Acetazolamide; arb.c.(IOC Confirm; 0 1) = ? |
|
01009
|
U-Acetazolamide; subst.c. = ? Ámol/l |
|
01010
|
Csf-Acetoacetate; subst.c. = ? Ámol/l |
|
01011
|
P-Acetoacetate; subst.c. = ? mmol/l |
|
01012
|
U-Acetoacetate; subst.c.(proc.) = ? mmol/l |
|
01013
|
P-Alkaline phosphatase, liver type; cat.c.(37 ░C; proc.) = ? Ákat/l |
|
01014
|
Amf-Acetyl-CoA acetyltransferase; cat.c.(37 ░C; proc.) = ? Ákat/l |
|
01015
|
Amf cells(cult.) prot.-Acetyl-CoA acetyltransferase; cat.cont.(37 ░C; proc.) = ? Ákat/kg |
|
01016
|
Chor.villus cell prot.-Acetyl-CoA acetyltransferase; cat.cont.(37 ░C; proc.) = ? Ákat/kg |
|
01017
|
Chor.villus cell(cult.) prot.-Acetyl-CoA acetyltransferase; cat.cont.(37 ░C; proc.) = ? Ákat/kg |
|
01018
|
Lkc prot.-Acetyl-CoA acetyltransferase; cat.cont.(37 ░C; proc.) = ? Ákat/kg |
|
01019
|
P-Acetyl-CoA acetyltransferase; cat.c.(37 ░C; proc.) = ? Ákat/l |
|
01020
|
Skin fibrobl. Prot.-Acetyl-CoA acetyltransferase; cat.cont.(37 ░C; proc.) = ? Ákat/kg |
|
01021
|
Skin fibrobl.(cult.) prot.-Acetyl-CoA acetyltransferase; cat.cont.(37 ░C; proc.) = ? Ákat/kg |
|
01022
|
U-N-Acetyl-L-cystathionine; subst.c. = ? Ámol/l |