ST08
Separation of a non-homogeneous liquid - liquid mixture
| Aim: To give an example of steam distillation: the extraction of fragrances from orange peel. |
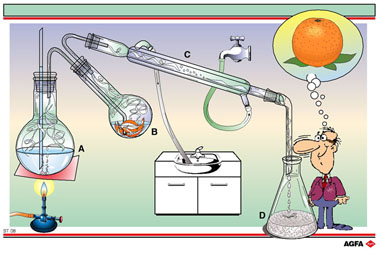
Illustration ST 08 shows the apparatus used to obtain terpenes (flavourings, fragrances and colourings) from orange peel by steam distillation. The first flask (A) generates steam that flows into a second flask (B). This flask contains orange peel covered with a layer of water. The steam extracts the terpenes out of the peel and these condense, together with the steam, in the condenser (C) and are collected (D). In principle this method is suitable for the separation of immiscible liquids.
In
such mixtures each component behaves independently of the others. This
type of mixture has a total vapour pressure that is equal to the sum of
the individual vapour pressures of the pure components.
Compared to the individual components, the total vapour pressure of the
mixture is thus higher, and it boils at a lower temperature. A liquid
with a high boiling point can be purified at a lower temperature by distilling
it with a solvent, preferably with a relatively high vapour pressure,
with which it does not mix. If water is used we speak of steam distillation.
This technique is used in particular for purification of liquids that
decompose at high boiling points.
General comment: to achieve optimal cooling in the condenser the water
should enter at the bottom and leave at the top.