ST01
Classification of mixtures according to the physical state
| Aim:
To show what a mixture is, and to give two examples of different
types of mixtures classified according to the distribution of their
components. |
In chemistry,
pure substances are investigated. This means substances that are distinguishable
from others by specific properties such as melting point, boiling point,
density etc.
In nature very few pure substances are found; normally the substances
are present in mixtures. Chemists have to separate these mixtures before
they can investigate the pure substances.
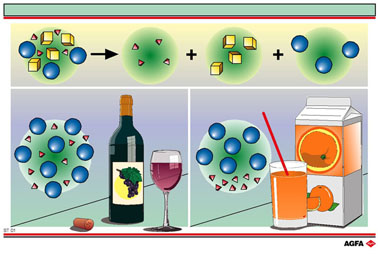
At the top of the illustration a mixture is shown that contains three
pure substances (components). We can classify mixtures according to the
physical state and the relative distributions of the components. These
physical states of these components can be solid, liquid or gas. We can
also distinguish between homogeneous and heterogeneous mixtures according
to the nature of their components.
‘Homogeneous’ means that all parts of the mixture have
the same composition. In a homogeneous mixture no separate parts or components
can be identified.
In
a solution this means that the solute concentration is the same throughout.
For a mixture of liquids this means that no liquid-liquid interfaces are
visible. For gas mixtures homogeneity means that the composition, density
and temperature are the same throughout.
Mixtures are given various names according to their physical states and
their composition. The table below gives a simple overview of the mixtures
and their common names.

On the left
of illustration ST 01, wine is given as an example of a homogeneous liquid
mixture (solution). Wine contains, among many other dissolved substances,
water and alcohol as major components. The building blocks (molecules)
of these two ingredients are shown symbolically on the illustration as
spheres and tetrahedrons.
On the right of the illustration orange juice, a heterogeneous mixture
(suspension), is depicted. In freshly squeezed fruit juice we find not
only liquid components (water + dissolved substances) but also fruit pulp
as a solid phase.