| Gibbs energy of repulsion (between two surfaces) |
| 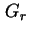 |
| Gibbs energy of repulsion due to electric effects |
|
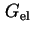 |
| Gibbs energy of repulsion per unit area of two parallel plates |
| 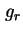 , ,
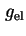 |
| Gibbs energy of attraction of two surfaces |
| 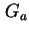 |
| Gibbs energy of attraction per unit area of two parallel plates |
| 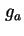 |
Van der Waals-London attraction constant between two molecules (
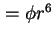 ) ) |
| 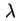 |
(Van der Waals-)Hamaker constant between semi-infinite flat plates, distance 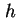 apart ( apart (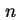 = number of molecules per unit volume) ( = number of molecules per unit volume) (
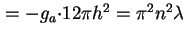 ) ) |
| 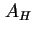 |
Retarded van der Waals constant between two molecules (
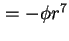 ) ) |
| 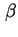 |
Retarded van der Waals constant between semi-infinite flat plates, distance 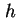 apart ( apart (
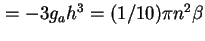 ) ) |
| 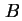 |
| Total Gibbs energy of interaction (and per unit area of two parallel plates) |
| 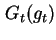 |
Disjoining pressure (
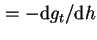 ) ) |
| 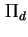 |