


Next: Enthalpy of wetting or
Up: MECHANICAL AND THERMODYNAMIC PROPERTIES
Previous: Surface tension, surface Helmholtz
Contents
Solid adsorbent/gas interface: characteristic thermodynamic quantities of adsorption
The following definitions refer to the solid/gas interface. Changes in enthalpy and entropy associated with adsorption are usually attributed to changes in the thermodynamic state of the adsorbate only. It should be borne in mind, however, that the measured changes include contributions from the perturbation of the adsorbent.
Differential energy of adsorption
When the addition of a differential amount d
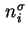 or d
or d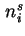 is effected at constant gas volume, the differential molar energy of adsorption of component
is effected at constant gas volume, the differential molar energy of adsorption of component 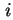 ,
,
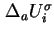 or
or
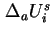 , is defined as:
, is defined as:
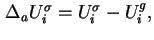
or
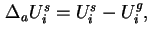
where the differential molar surface excess energy,
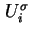 , is given by 18
and the differential molar interfacial energy,
, is given by 18
and the differential molar interfacial energy, 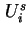 , by
, by
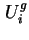 is the differential molar energy of component
is the differential molar energy of component 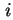 in the gas phase, i.e.
in the gas phase, i.e.
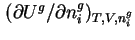 .
.
Differential enthalpy of adsorption
When the addition of the differential amount d
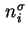 or d
or d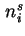 is effected at constant pressure
is effected at constant pressure 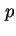 , the differential molar enthalpy of adsorption,
, the differential molar enthalpy of adsorption,
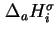 or
or
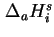 , also called the isosteric enthalpy of adsorption (
, also called the isosteric enthalpy of adsorption (
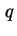 st)19 is defined as20
st)19 is defined as20
where
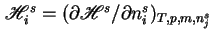 , and
, and 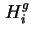 is the partial molar enthalpy of component
is the partial molar enthalpy of component 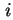 in the gas phase, i.e.
in the gas phase, i.e.
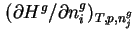 .
.
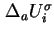 and
and
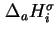 are related by the equation:
are related by the equation:
and the same applies to the difference between
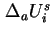 and
and
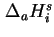 when
when 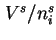 is negligibly small compared with
is negligibly small compared with 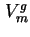 .
.
When an excess
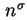 of a single adsorptive is adsorbed on a surface initially free of adsorbate species, the molar integral energy and molar integral enthalpy of adsorption are given by
of a single adsorptive is adsorbed on a surface initially free of adsorbate species, the molar integral energy and molar integral enthalpy of adsorption are given by
Experimental calorimetric methods have to be analysed carefully to establish the appropriate procedure for deducing a particular energy or enthalpy of adsorption from measured data. The isosteric enthalpy of adsorption is usually calculated from adsorption isotherms measured at several temperatures by using the equation
where 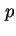 is the equilibrium partial pressure of the adsorptive when an amount
is the equilibrium partial pressure of the adsorptive when an amount
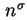 is adsorbed at a temperature
is adsorbed at a temperature 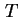 .
.
Standard thermodynamic quantities. For different purposes
it may be convenient to define standard changes of a thermodynamic quantity on adsorption in two alternative ways:
- (i)
- the change of the thermodynamic quantity on going from the standard gas state to the adsorbed state in equilibrium with gas at a partial pressure (fugacity) of
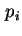
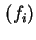 . Such quantities are sometimes called `half-standard' quantities.
. Such quantities are sometimes called `half-standard' quantities.
- (ii)
- the change of the thermodynamic quantity on going from the standard gas state to a defined standard condition of the adsorbed state.
It must always be stated clearly which of these conventions is being followed.
The standard Gibbs energy of adsorption is thus:
where
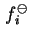 is the fugacity of
is the fugacity of 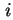 in the standard gas state and
in the standard gas state and 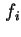 the fugacity of gas in equilibrium with the (standard) adsorbed state. For most practical purposes the fugacity may be replaced by the (partial) pressure.
the fugacity of gas in equilibrium with the (standard) adsorbed state. For most practical purposes the fugacity may be replaced by the (partial) pressure.
If unit pressure is chosen for the standard gas state, this may be written
while if vapour in equilibrium with pure liquid 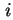 is chosen21, then
is chosen21, then
Similarly, the standard differential molar entropy of adsorption is given by
where
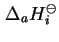 is the standard differential molar enthalpy of adsorption.
is the standard differential molar enthalpy of adsorption.
The standard integral molar entropy of adsorption is
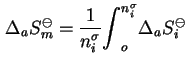
d
The above definitions refer to equilibrium conditions, and their applicability in regions where adsorption hysteresis occurs is open to doubt.



Next: Enthalpy of wetting or
Up: MECHANICAL AND THERMODYNAMIC PROPERTIES
Previous: Surface tension, surface Helmholtz
Contents
2002-09-05
![]() or d
or d![]() is effected at constant gas volume, the differential molar energy of adsorption of component
is effected at constant gas volume, the differential molar energy of adsorption of component ![]() ,
,
![]() or
or
![]() , is defined as:
, is defined as:
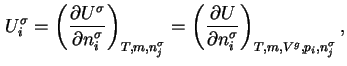
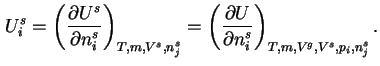
![]() is the differential molar energy of component
is the differential molar energy of component ![]() in the gas phase, i.e.
in the gas phase, i.e.
![]() .
.
![]() or d
or d![]() is effected at constant pressure
is effected at constant pressure ![]() , the differential molar enthalpy of adsorption,
, the differential molar enthalpy of adsorption,
![]() or
or
![]() , also called the isosteric enthalpy of adsorption (
, also called the isosteric enthalpy of adsorption (
![]() st)19 is defined as20
st)19 is defined as20
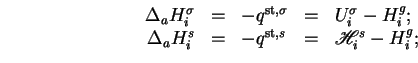
![]() and
and
![]() are related by the equation:
are related by the equation:
![]() of a single adsorptive is adsorbed on a surface initially free of adsorbate species, the molar integral energy and molar integral enthalpy of adsorption are given by
of a single adsorptive is adsorbed on a surface initially free of adsorbate species, the molar integral energy and molar integral enthalpy of adsorption are given by
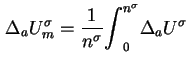 d
d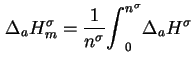 d
d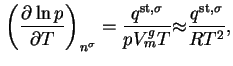
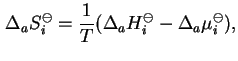
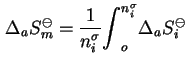 d
d