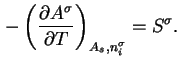The surface tension is related to the derivative of the Helmholtz energy by the equations
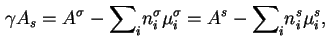
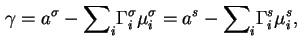
Note: Only when
![]() is
is ![]() equal to the
surface (excess) Helmholtz energy per unit area. In general, for a multicomponent system it is not possible to define either an interfacial layer, or a Gibbs surface, for which this condition is satisfied. However, it is satisfied automatically when the system exhibits an adsorption azeotrope at which all the
equal to the
surface (excess) Helmholtz energy per unit area. In general, for a multicomponent system it is not possible to define either an interfacial layer, or a Gibbs surface, for which this condition is satisfied. However, it is satisfied automatically when the system exhibits an adsorption azeotrope at which all the
![]() are zero.
are zero.
For a one-component system, treated in terms of a Gibbs surface it is always possible to choose this surface so that
![]() , so that the surface tension is equal to the value of
, so that the surface tension is equal to the value of
![]() relative to this surface; on the other hand
relative to this surface; on the other hand
![]() must always be positive for an interfacial layer so that
must always be positive for an interfacial layer so that ![]() and
and ![]() can never be equated.
can never be equated.
The surface excess entropy is given by