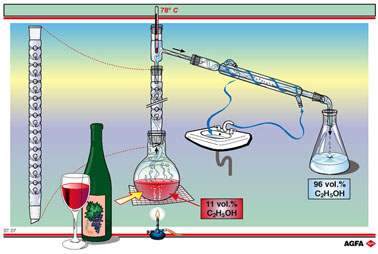
ST07
Separation of liquid - liquid mixtures (solutions)
| Aim:
To show the use of fractional distillation for the separation of
liquid - liquid mixtures in which the boiling points of the components
are similar. |
To achieve
a complete separation by distillation the vapour that condenses first
may only contain particles (molecules) of the component with the lowest
boiling point (the more volatile component in the mixture). This condition
can only be satisfied when the boiling points of the two-components that
are to be separated are very different. A rule of thumb is that for a
normal distillation the boiling points must differ by at least 25 °C.
Even if this condition is met the distillate will contains particles (molecules)
of the less volatile component and the separation is never complete.
In practice use is made of fractional distillation, a technique that gives
a better separation. With this technique it is possible to separate mixtures
of liquids whose boiling points are considerably less than 25°C apart.
Again however this technique does not give perfect separation.
This more effective separation is achieved by placing a fractionating
column (a Vigreux column) between the distillation flask and the (Liebig)
condenser. This column is a tube filled with glass beads or rods, or a
tube in which the walls have many inward-pointing indentations (see illustration).
Higher up the column the temperature is lower, and the vapour-mixture
condenses. The rising vapour must pass through the falling drops of distillate
to reach the condenser. Because of this close contact between the rising
vapour and the falling condensate only the particles (molecules)with the
lower boiling point can rise up the column. As a result, the higher up
the column we go, the richer the vapour is in molecules of the most volatile
component.
Theoretical background :
If the volatile components that make up the mixture follow Raoult’s
law which states that
Psoln=Xsolvent P0 solvent
Where
P
Xsolvent is the mole fraction of the solvent
P0 solvent is the vapour pressure of the pure solvent
then the total vapour pressure above a mixture is given by
Ptot = X1P01 + X2P02
The
contribution of every component to the total vapour pressure is directly
proportional to the mole fraction X of this component multiplied by its
vapour pressure in a pure state, P0. This applies to an ideal
mixture which can thus be separated effectively by distillation. The efficiency
of separation increases as the difference in boiling points increases.
The figure below shows the change in composition for a mixture of two
components that obey Raoult’s law. The yaxis gives the boiling points
of the pure components of the mixture (T1 and T2).
The two points are joined by two curves that show the change in composition
of the liquidmixture (bottom curve) and the vapour-mixture (top curve).
The x-axis represents the composition of the mixture.
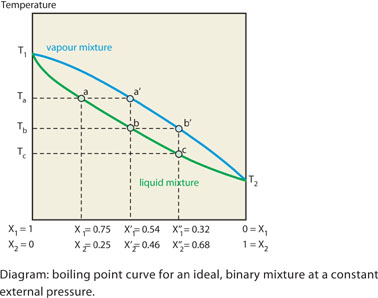
A
mixture in which component 1 has a mole fraction equal to 0.75 (X1
= 0.75) and component 2 equal to 0.25 (X2 = 0.25) boils at
a temperature Ta. The lower curve shows the composition of the liquid
phase. For the mixture under discussion this is represented by the point
a on the curve. The vapour phase that is in equilibrium with this has
a composition a’ for which the mole fractions of the two components
are X1’ = 0.54 and
X2’ = 0.46 respectively. From this we can see that the
vapour is richer in the more volatile component, 2.
If vapour with this composition is condensed, then the mixture obtained
is enriched in the most volatile component. This represents one step in
the distillation process. The vapour phase that is in equilibrium with
the liquid of composition b has a composition b’ with the following
mole fractions: X1’’= 0.32 and X2’’
= 0.68. On cooling, the resulting liquid has this composition c. This
is the second distillation step. As a result, the mole fraction of the
most volatile component (component 2) increases further with every distillation
step. In fractional distillation the number of distillation steps is much
greater than in normal distillation.
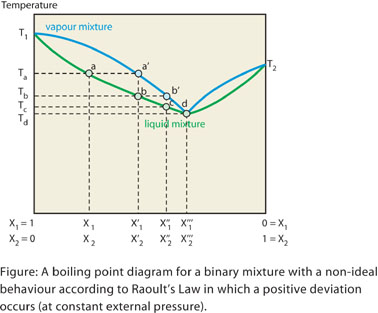
If Raoult’s law is not obeyed, deviations arise. In the case of
a positive deviation the vapour pressure of the binary mixture is higher
than that predicted by Raoult’s law. In this case the mixture boils
at a lower temperature.
Negative deviations, on the other hand, reduce the vapour pressure and
raise the boiling point.
For positive deviations it can sometimes happen that the boiling point
of the mixture is lower than that of the more volatile component. Under
such circumstances a minimum in
the boiling point curve will be present for a certain composition.
This mixture is termed a positive "azeotrope", which means literally
"cannot be changed by boiling". For this minimum-boiling-point
mixture the composition of the liquid phase is indeed identical to that
of the gas phase.
This means that the mixture at this point behaves as a pure substance.
Moreover since the composition of the vapour phase is identical to that
of the liquid phase the mixture cannot be separated further by distillation.
The diagram below shows a boiling point curve for a two-component mixture
where the positive deviations to Raoult’s law lead to a minimum
boiling point.
An example of this is the water / ethanol mixture, which at a certain
composition shows a lower boiling point (78.2 °C) than pure ethanol
(T2 = 78.3 °C). At this point the water / ethanol mixture
contains 4% w/w of water (3% v/v, X1’’’ =
0.09), and 96% w/w ethanol (97% v/v, X2’’’
= 0.91).
Negative deviations from Raoult’s law sometimes lead to a maximum
in the boiling point of the binary mixture. For such a mixture the composition
of the two phases is once again equal. This mixture is a negative azeotrope.
An example is the mixture of water / 20.2% w/w hydrogen chloride, with
a boiling point of 108.6 °C, which is higher than that of pure water
(T1 = 100 °C).
Negative deviations from Raoult’s law arise when the interactions
between dissimilar molecules are stronger than those between similar molecules.
The mixing process is accompanied by generation of heat (an exothermic
mixture).
Positive deviations from Raoult’s law can have two possible causes:
(1) the interactions between dissimilar molecules are weaker and the mixing
process is endothermic; or (2) the molecular disorder in the mixture is
less than that envisaged by Raoult’s law. This explains why some
exothermic mixtures, such as the ethanol / water system, can nevertheless
exhibit positive deviations.
In the example of illustration ST07 the starting point is wine. Wine has
an ethanol content of something more than 10%. Fractional distillation
gives a mixture containing 96% w/w ethanol and 4% w/w water. To obtain
100% w/w ethanol the mixture must be treated further with a drying agent
such as CaCl2.