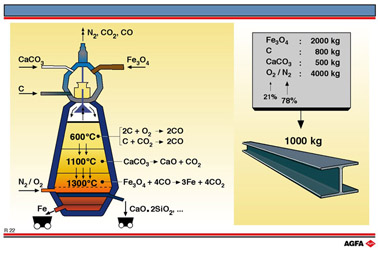
R22 Electrochemistry in the blast furnace
Aim: To illustrate the electrochemical processes which occur between gases and solids in a blast furnace |
The production of iron in
blast furnaces has been of major economic importance for centuries. Illustration R22 shows
the processes involved. The reduction of iron ore (oxides of iron(II), iron(III), silicon
and other metals)
to iron with coke can be regarded as an example of a redox reaction. A blast furnace is
designed to realise the following overall reaction:
![]()
For the sake of investigating the energetics of this reaction, it can be regarded as
taking place in the presence of water. Under such conditions the reaction can be presented
by the following half-reaction equations :
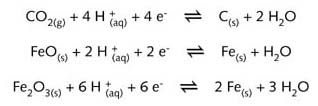
Although the reduction of iron(II) and iron(III) oxides and the oxidation of coke are
self-sustaining reactions, the activation energy threshold for both is high. In reality
the processes are high temperature gas-solid reactions taking place in the absence of
water. In view of the high activation energy threshold, the temperatures in the reaction
zones of the blast furnace need to be very high.
Such high temperatures are achieved by blowing hot air over the coke, which is thereby
initially oxidised exothermically to carbon dioxide:
![]()
![]()
The carbon monoxide reduces the iron oxides exothermically, the iron is formed dropping to
the bottom of the furnace, where the temperature is high enough to melt it.
![]()
A pool of molten iron forms on the bottom of the furnace. Formation of a molten slag results from the limestone (which is included in the charge together with the iron ore and coke) dissociating to form calcium oxide and carbon dioxide
![]()
and then combining with the silicon oxide and impurities from the ore.
This slag trickles down to the bottom and, being less dense, forms a layer
on top of the molten iron.
The iron and slag are tapped off every few hours. A modern furnace makes
3000 tons of iron a day.