M05 Carbon-14 decay
Aim: To explain the changes which occur in the nucleus during the radioactive decay of 14C. |
Isotopes of carbon
Carbon has three isotopes, (isotopes are nuclides of the same element
containing different numbers of neutrons in the nucleus) :
![]() has 8 neutrons and is a radioactive isotope and its slow rate of decay
enables it to be used in the dating of plant and animal matter in so-called
radiocarbon dating;
has 8 neutrons and is a radioactive isotope and its slow rate of decay
enables it to be used in the dating of plant and animal matter in so-called
radiocarbon dating;
![]() (so called C-12) has 6 neutrons, and is the most common isotope;
(so called C-12) has 6 neutrons, and is the most common isotope;
![]() has 7 neutrons and has a natural abundance of 1.1% of
has 7 neutrons and has a natural abundance of 1.1% of ![]() ,
but its low natural abundance is utilised in the analysis of organic molecules
by
,
but its low natural abundance is utilised in the analysis of organic molecules
by ![]() NMR spectroscopy.
NMR spectroscopy.
Carbon-14 is continuously formed in the upper atmosphere by the collision
of neutrons with nitrogen atoms:
![]()
![]()
The ratio of 14C/12C in the air remains constant,
due to the fact that the rate of production of C-14 by the above reaction
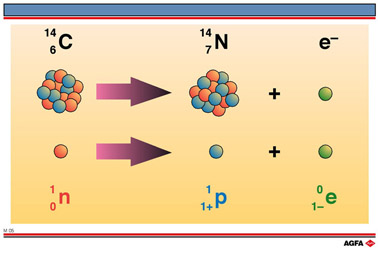
is equal to the rate of decay. In the nucleus of ![]() a neutron splits into a proton and an electron:
a neutron splits into a proton and an electron:
![]()
Radiocarbon dating
Plants assimilate CO2, which has this same ratio 14C/12C,
during photosynthesis. Any living creature which consumes plants thus takes in the same
ratio of C-14 /C-12. The activity of carbon in living tissue is thus 0.007µCi per kg.
When a plant or animal dies, however, the assimilation of carbon-14 stops. The quantity of
C-14 decays with time, and the radioactivity that remains can be used to calculate the age
of the plant or animal material. After 5,750 years half of the radioactive C-14 has
decayed (this time is known as the half-life). Fossils and other materials of natural
origin (e.g. the parchment of the Dead Sea Scrolls) have been dated using this method. The
method is most accurate for ages up to 5,000 years, but it has been used for objects up to
45,000 years old