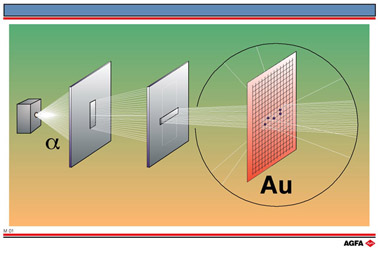
M01 - M02 Atomic model according to Rutherford
Aim: To explain Rutherford’s scattering experiment |
The idea put
forward by the Greek philosopher Democritus, that the atom (Greek: atomos
, indivisible) was the smallest particle of which matter was made, gave
way at the beginning of the twentieth century to the idea that atoms were
themselves made of even smaller particles: a very small positively charged
central nucleus surrounded by negatively charged electrons.
Rutherford’s scattering experiment (1909) played a crucial role in
this development. His colleagues fired a beam of ![]() -particles
(helium nuclei) at a film of gold foil and found that whilst the majority
of particles passed straight through, a small fraction ( about 1 in 8000
) were deflected through a large angle. To explain these observations,
Rutherford suggested that since
-particles
(helium nuclei) at a film of gold foil and found that whilst the majority
of particles passed straight through, a small fraction ( about 1 in 8000
) were deflected through a large angle. To explain these observations,
Rutherford suggested that since
![]() -particles are positively
charged such deflection could only be caused by the particles coming close
to a concentrated region of positive charge.
-particles are positively
charged such deflection could only be caused by the particles coming close
to a concentrated region of positive charge.
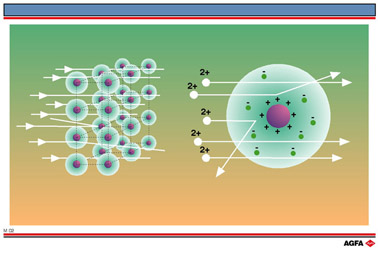
Only a very small fraction of
the ![]() -particles were
deflected, so he concluded that the region of positive charge which caused
the scattering would only occupy a small part of the atom. From this he
deduced that the atoms in the metal foil consisted of a central positive
nucleus composed of protons, where the mass of the atom was concentrated.
-particles were
deflected, so he concluded that the region of positive charge which caused
the scattering would only occupy a small part of the atom. From this he
deduced that the atoms in the metal foil consisted of a central positive
nucleus composed of protons, where the mass of the atom was concentrated.
Other models
which were current at that time (including Thomson’s “plum pudding”
model, which saw the atom as a sphere of positive electricity in which
negative electrons were embedded) could not account for the deflection
of the ![]() -particles.
-particles.