E09 Applications of the equilibrium law : a change in concentration of one component
|
Aim: To show how, as a result of the equilibrium
law, the equilibrium mixture can be changed by changing the concentration
of one component. |
It is obvious, that such a simple fundamental law has many interesting and useful applications:
- If the equilibrium constant of a specific reaction at particular temperature is known, one can predict (calculate) for known reactant concentrations the concentrations applying at equilibrium. The degree of conversion can also be calculated, but not the equilibration time.
- If the initial composition
of an equilibrium reaction mixture and the equilibrium concentration
of one of the components are known, then the other equilibrium concentrations
can be calculated and also the Kc-value of the reaction
at that particular
temperature. - The equilibrium law
can be used in industrial chemical reactions to increase the yield of
a reaction which does not go to completion by varying the initial concentrations
of the reactants.
These application possibilities will be discussed at greater length later in this text.
We could even agree that a change in temperature changes the equilibrium law itself: for each reaction temperature a different equilibrium constant applies. Its influence clearly merits separate discussion.
The only
other factor, apart from reaction temperature,which can change the equilibrium
mixture is a change in concentration of one of the components or all of
the components at the same time.
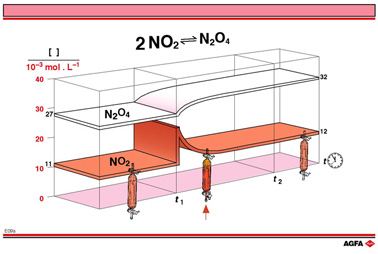
In illustration E09a, the effect of a change in the NO2- concentration
on the NO2-dimerization reaction is shown. The concentrations
of the reactant, NO2, and product, N2O4
are plotted as a function of time. The initial situation corresponds with
the equilibrium of experiment A in the table on illustration E06.
The initial equilibrium concentrations of NO2 and N2O4are
0.011 mol/L and 0.027 mol/L respectively. The brown band shows a doubling
of NO2-concentrations at time t1, by adding
NO2 from outside the closed system.
Between time t1 and the achievement of a new equilibrium
mixture at time t2 there is no equilibrium, the NO2-
and N2O4-concentrations in the reaction mixture
changing as shown in illustration E09a until new equilibrium concentrations
are achieved. The new equili-brium concentrations for NO2 and
N2O4 are 0.012 mol/L and 0.032 mol/L respectively.
After the concentration change, the concentrations of the components vary
in the closed system.
This can seem strange at first : a rapid doubling in NO2- concentration
leading to a strong increase in N2O4- concentration
(N.B. this specific case may not be freely generalized).
These sometimes unexpected changes is concentration
can be explained in terms of the equilibrium law. In illustration E09b,
use is made of the term reaction quotient Qc, to help
explain this. The reactant quotient of an equilibrium
reaction Qc is analogous to Kc, differing in the
use of the actual concentrations of the components present at a given
time, rather than the equilibrium concentrations of the components.
In the case of the NO2-dimerization reaction, the appropriate expressions for Qc and Kc are:
![]()
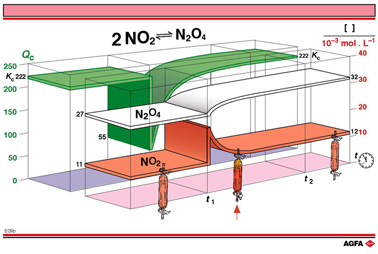
At time t1, a
doubling of the NO2-concentration yields a Qc value of
about 55 i.e. (0.027)/(0.0022)2. This as a quarter of the Kc-value
for this equilibrium at the same temperature. Thus Qc < Kc.
According to the equilibrium law, Qc will increase until Qc
equals Kc i.e. 222. It is this change in Qc with
time, which can be followed as the green band in illustration E09b.
To attain the Kc value, the numerator has to increase by an amount x
to (0.027 + x). The denominator has to decrease correspondingly by 2x to (0.011 - 2x), as
2 molecules of NO2 are consumed for each molecule of N2O4
formed.
The new equilibrium concentrations, indicated by [ ]’e, can be calculated using the equilibrium law and the Kc value by solving the equation:
![]()
In the following table, quantititive results are summarized, which are useful in the interpretation of illustrations E09a and E09b.
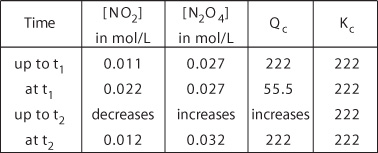
The new equilibrium concentrations 0.012 mol/L for NO2 and 0.032 mol/L for N2O4,
when inserted in the expression for Kc give a Kc
value of 222.
The result of this intervention, an be explained qualitatively as follows:
“If change occurs in the concentration of a component
in an equilibrium mixture, the system will tend to adjust itself so as to annul, as far as
possible, the effect of that change”.
This rule of thumb is known as “Le Chatelier’s Principle” or the principle
of “least stress”.
BEWARE
The disturbance in a chemical equilibrium is not completely counteracted, as appears it to
be in the case of phase-equilibria. The NO2-concentration increase is
counteracted as much as possible, the initial 0.022 mol/L being reduced to 0.012 mol/L.
However, this is still higher than the initial value of 0.011 mol/L. The disturbance has
an interesting result, the position of the equilibrium being shifted to the right,
although the degree of conversion has decreased. For the sake of completeness, it should
be repeated that a closed system at chemical equilibrium is being considered in which the
reaction temperature remains constant. That this disturbance is almost completely
counteracted by adaptions in the reaction mixture is thanks to the fairly large value of Kc
, in this case. In the case of reactions with much smaller Kc values
(e.g. weak acids) the amount of counteraction is much smaller.